sodium benzoate & methyl benzoate
In sodium benzoate the two carbon oxygen bonds are of the same length, whereas
in methyl benzoate these lengths are different.
Suggest why this is the case, illustrating your answer with appropriate
diagrams.
Can someone please explain to me the answer to this question
in methyl benzoate these lengths are different.
Suggest why this is the case, illustrating your answer with appropriate
diagrams.
Can someone please explain to me the answer to this question
Recall that the C-C bond lengths in benzene are all the same due to resonance of the pi system in the ring and not double - single - double etc.
Similar effect here with the benzoate ion:
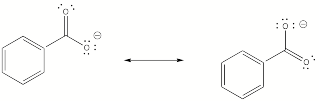
Similar effect here with the benzoate ion:
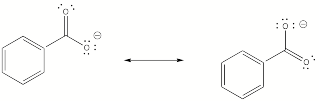
Original post by EierVonSatan
Recall that the C-C bond lengths in benzene are all the same due to resonance of the pi system in the ring and not double - single - double etc.
Similar effect here with the benzoate ion:
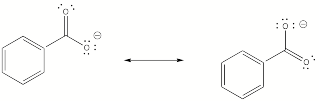
Similar effect here with the benzoate ion:
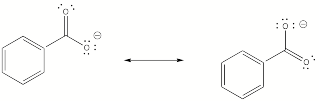
I'm sorry but i fail to understand

What is resonance of the pi system? I know that benzene is a hybrid which means it neither has single bonds, neither does it have double bonds
Original post by aqua05
??
I'm sorry but i fail to understand
What is resonance of the pi system? I know that benzene is a hybrid which means it neither has single bonds, neither does it have double bonds
I'm sorry but i fail to understand

What is resonance of the pi system? I know that benzene is a hybrid which means it neither has single bonds, neither does it have double bonds
Okay, so you know that benzene doesn't have alternating single and double bonds. This is because the electrons are able to move around the ring and do not stay in the same place, that's why we draw a circle instead of double and single bonds

The COO- group is just the same draw one 'state' (like in the left of the diagram above)and it has a C=O bond and a C-O- bond. Like in benzene these electrons are free to move and so can switch (to the other state such as the right structure on the diagram). So there are no double and single bond - just a uniform C-O bond inbetween double and single.
Original post by EierVonSatan
Okay, so you know that benzene doesn't have alternating single and double bonds. This is because the electrons are able to move around the ring and do not stay in the same place, that's why we draw a circle instead of double and single bonds 
The COO- group is just the same draw one 'state' (like in the left of the diagram above)and it has a C=O bond and a C-O- bond. Like in benzene these electrons are free to move and so can switch (to the other state such as the right structure on the diagram). So there are no double and single bond - just a uniform C-O bond inbetween double and single.

The COO- group is just the same draw one 'state' (like in the left of the diagram above)and it has a C=O bond and a C-O- bond. Like in benzene these electrons are free to move and so can switch (to the other state such as the right structure on the diagram). So there are no double and single bond - just a uniform C-O bond inbetween double and single.
Woww:-) actually understood it.,
Just one thing.. methy benzoate cannot change its state so has alternating lengths.right??
And in the exam how should i answer it? I mean I can't write about benzene ring & stuff:s
Original post by aqua05
Woww:-) actually understood it.,
Just one thing.. methy benzoate cannot change its state so has alternating lengths.right??
Just one thing.. methy benzoate cannot change its state so has alternating lengths.right??
The electrons in the ester aren't free to move because the two electrons (which can move in the benzoate ion) are being shared with the carbon.
And in the exam how should i answer it? I mean I can't write about benzene ring & stuff:s
If you draw the diagram above (it asks you to use diagrams) then say the electrons are free to move between the two forms - creating an average bond length.
Original post by EierVonSatan
The electrons in the ester aren't free to move because the two electrons (which can move in the benzoate ion) are being shared with the carbon.
If you draw the diagram above (it asks you to use diagrams) then say the electrons are free to move between the two forms - creating an average bond length.
If you draw the diagram above (it asks you to use diagrams) then say the electrons are free to move between the two forms - creating an average bond length.
thanks so much for your help!

Got it

Quick Reply
Related discussions
- Amount of substance chemistry
- Indicator colour question.
- Organic chemistry questions
- [Chemistry] Equilibrium Question
- Organic chemistry help
- Edexcel A-Level Chem Paper 1 Advanced Inorganic and Physical Chemistry [Exam Chat]
- Boiling point of halogenoalkanes
- Why is1-methylpentane not an isomer of Hexane
- Help chemistry
- OCR A-Level Chemistry B Paper 2 (H433/02) - 19th June 2023 [Exam Chat]
- chem isomer as help?
- Chemistry naming alkanes draw structure
- Nucleophilic substitution - Need help
- Reaction of Alcohols
- Skeletal Formulae
- A level chemistry optical isomerism MC questions
- is but-1-ene the same as butene
- AQA A-level Biology 7402 - Paper 2 - 13th June 2019
- Chemistry question- enthalpy of formation
- how do i suggest the formula for sodium chlorate (vii) ??
Latest
Trending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these products



