Redox Equilibria
Given the following redox reactions:

How many moles of iron (II) ethandioate FeC2O4 are oxidised by 1 mol of dichromate Cr2O72–(aq)?
(I thought the answer was 2. However the mark scheme says 3, and does not explain why.)
How many moles of iron (II) ethandioate FeC2O4 are oxidised by 1 mol of dichromate Cr2O72–(aq)?
(I thought the answer was 2. However the mark scheme says 3, and does not explain why.)
 I dont know, sorry
I dont know, sorry 
I would have said two, as you've got 6e- on the LHS, and 3e- on the right, so I would have doubled the number of moles on the RHS, giving 2 moles...
sorry, that is of no use what so ever...
best to ask teacher tomorrow at college perhaps?
unless someone else can shed some light on this. im curious now, i wonder what we're doing wrong??

Original post by HeatherJarvo I dont know, sorry
I dont know, sorry 
I would have said two, as you've got 6e- on the LHS, and 3e- on the right, so I would have doubled the number of moles on the RHS, giving 2 moles...
sorry, that is of no use what so ever...
best to ask teacher tomorrow at college perhaps?
unless someone else can shed some light on this. im curious now, i wonder what we're doing wrong??
 I dont know, sorry
I dont know, sorry 
I would have said two, as you've got 6e- on the LHS, and 3e- on the right, so I would have doubled the number of moles on the RHS, giving 2 moles...
sorry, that is of no use what so ever...
best to ask teacher tomorrow at college perhaps?
unless someone else can shed some light on this. im curious now, i wonder what we're doing wrong??

Thank you. I asked my teacher, he said the mark scheme got it wrong.
Original post by Gattsu
Thank you. I asked my teacher, he said the mark scheme got it wrong.
that's a scary thought isn't it? It's possible to lose marks on the exam by getting the answer correct.... eek!!
Original post by Gattsu
Given the following redox reactions:

How many moles of iron (II) ethandioate FeC2O4 are oxidised by 1 mol of dichromate Cr2O72–(aq)?
(I thought the answer was 2. However the mark scheme says 3, and does not explain why.)
How many moles of iron (II) ethandioate FeC2O4 are oxidised by 1 mol of dichromate Cr2O72–(aq)?
(I thought the answer was 2. However the mark scheme says 3, and does not explain why.)
Original post by HeatherJarvo I dont know, sorry
I dont know, sorry 
I would have said two, as you've got 6e- on the LHS, and 3e- on the right, so I would have doubled the number of moles on the RHS, giving 2 moles...
sorry, that is of no use what so ever...
best to ask teacher tomorrow at college perhaps?
unless someone else can shed some light on this. im curious now, i wonder what we're doing wrong??
 I dont know, sorry
I dont know, sorry 
I would have said two, as you've got 6e- on the LHS, and 3e- on the right, so I would have doubled the number of moles on the RHS, giving 2 moles...
sorry, that is of no use what so ever...
best to ask teacher tomorrow at college perhaps?
unless someone else can shed some light on this. im curious now, i wonder what we're doing wrong??

Couldnt figure out how you form iron (II) ethandioate FeC2O4 in the first place !?
Original post by arvin_infinity
Couldnt figure out how you form iron (II) ethandioate FeC2O4 in the first place !?
Why can you not form it?
(edited 12 years ago)
Original post by thegodofgod
Why can you not form it?
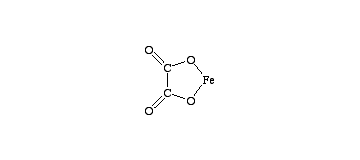
Oh no..I meant , how you form it according the the equation given to you
Original post by arvin_infinity
Oh no..I meant , how you form it according the the equation given to you
Fe2+ + (COOH)2 --> [Fe(COO)2] + 2H+
Original post by charco
Fe2+ + (COOH)2 --> [Fe(COO)2] + 2H+
COOH is not given to us !!!
I guess I misunderstood the question

Quick Reply
Related discussions
- AS Chemistry Paper 1 2022
- (AQA AS Chemistry) Redox reactions - help!
- chemistry a level
- Chem alevel help
- a level redox question
- chemistry as level equilibria help
- Hard friction question
- National 5 Chemistry
- Ocr a level chem
- Tips for physical A level chemistry OCR A?
- Redox reactions between a free element and a compound
- Redox Reactions
- A level chemistry Redox help please.
- Game Theory question - URGENT HELP NEEDED!
- Is the reaction between an amino acid and HCl a neutralisation or redox
- (AS AQA Chemistry) Redox reactions - general question
- Ocr gateway a chemistry as level paper 2021 paper 2
- Redox and balancing
- Chemsheet transition metal answers??
- A level Chemistry help
Latest
Last reply 1 minute ago
KPMG Audit Apprenticeship 2024Last reply 3 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 3 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 4 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1251
Last reply 4 minutes ago
Official University of Edinburgh Offer Holders Thread for 2024 entryLast reply 7 minutes ago
Official: University of Leicester A100 2024 Entry ApplicantsLast reply 8 minutes ago
can any people who are studying at exeter's penryn campus please answer this?Last reply 9 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 9 minutes ago
Can I drop out of college once I have an unconditional uni offer?Last reply 11 minutes ago
Accomodation in UK for Royal Holloway student - Sep 2024Last reply 15 minutes ago
GCSE AQA FRENCH SPEAKING - General conversation and what theme to choose?Last reply 15 minutes ago
BAE systems degree apprenticeships September 2024Last reply 16 minutes ago
LSE Undergraduate Politics and IR 2024Posted 19 minutes ago
Chances of switching from LSE Acc and Fin to Econ and Maths or Fin Maths and StatsLast reply 19 minutes ago
Official: Aston University A100 2024 Entry Applicant threadMedical Schools
1120
Last reply 19 minutes ago
Amazon Project management apprenticeship 2024



