Titation between Manganate and iron(II) ions - Electrode Potentials
Question:

Values from Data Booklet:
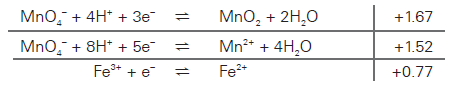
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?

Values from Data Booklet:
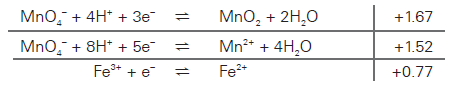
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?
(edited 12 years ago)
Original post by Zishi
Question:

Values from Data Booklet:
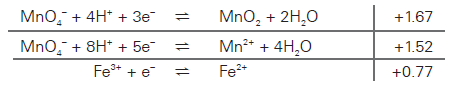
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?

Values from Data Booklet:
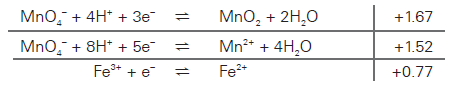
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?
I'm guessing your exam board uses the clockwise rule? Edexcel use the anti-clockwise rule (most negative on top of list, most positive at the bottom). Therefore, anything on the left will react with ANYTHING above it and to the right, I apologise if this is confusing.
Original post by Zishi
Question:

Values from Data Booklet:
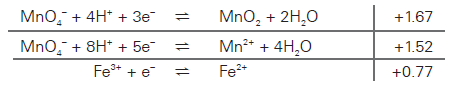
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?

Values from Data Booklet:
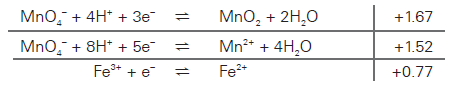
What I don't understand is why the Permanganate ions will reduce to Mn(II) ions instead of MnO2, as stated in the mark scheme? Is it because of the fact that we don't get black/brown solid(MnO2) at the end of the titration?
The more positive a reduction potential value is, the better oxidising agent it is. Therefore, it will become reduced more easily, and thus gain electrons more easily.
I think it's just because MnO4- is a very strong oxidising agent (compare to the likes of K2Cr2O7), and thus will become reduced very easily to Mn2+, instead of Mn4+ in MnO2.
Quick Reply
Related discussions
- Redox titration calculations- help! =S
- Electrochemistry
- A level Chemistry help
- Etha
- What is the ionic charge and formulae for nickel(II) manganate(VII)?
- Need Help on an Electrochem Q
- Catalyst: iodide and peroxodisulphate ions
- Inorganic Chemistry (AQA) - A level
- Electrochemistry help
- Electrochemical cell diagram: when do you add H+ to the solution? a level chem
- Chem a level - electrochemistry
- Redox and balancing
- A-level Chemistry Study Group 2022-2023
- Chemistry
- Transition Metal Ions
- AQA A Level Chemistry Electrochemistry
- A level physics question
- Alevel chemistry
- Chemisty Calculation A2 OCR Trends and patterns jan09
- AQA A Level Chemistry Paper 3 7405/3 - 23 Jun 2022 [Exam Chat]
Latest
Last reply 3 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 8 minutes ago
NICS Staff Officer and Deputy Principal recruitment 2022 2023Last reply 13 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1292
Last reply 14 minutes ago
The Official King's College London Applicants for 2024 Entry ThreadLast reply 17 minutes ago
Woodhouse College applicants 2024Last reply 17 minutes ago
BAE systems degree apprenticeships September 2024Last reply 19 minutes ago
OCR A-LEVEL PSYCHOLOGY PAPER 3 (H567/03) - 3rd June [Exam Chat]Last reply 21 minutes ago
OCR A-LEVEL PSYCHOLOGY PAPER 2 (H567/02) - 22nd May [Exam Chat]Last reply 22 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 24 minutes ago
Official: University of Bristol A100 2024 Entry Applicants



