Unless I'm mistaken, the R group specifically refers to some sort of alkyl chain or hydrogen.
Although a quick Google says it can sometimes mean nitrogen, halogen or oxygen etc.
Although a quick Google says it can sometimes mean nitrogen, halogen or oxygen etc.
so why is ketone the answer here 
Original post by Wunderbarr
Unless I'm mistaken, the R group specifically refers to some sort of alkyl chain or hydrogen.
Although a quick Google says it can sometimes mean nitrogen, halogen or oxygen etc.
Although a quick Google says it can sometimes mean nitrogen, halogen or oxygen etc.
Original post by All rounder
so why is ketone the answer here 
You agree that a ketone functional group is this:
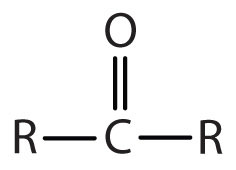
R must be either an alkyl group or aromatic group. In either case, you're going to have CARBON atoms bonded to the C=O.
Spoiler
Wunderbarr is slightly mistaken.
It CANNOT be a Nitrogen or a Halogen or a Hydrogen or an Oxygen. If R was any of these, the functional group has changed; it's no longer a ketone.
If it's a Nitrogen, then it's an Amide/Amine
If it's a Halogen, then it's an Acyl Halide
If it's a Hydrogen, then it's an Aldehyde
If it's an Oxygen, then it's an Ester/Carboxylic Acid.
I hope you do know what those functional groups all are...
There's no ketone in the diagram, the 2 C=O group is attached to N-H; NH-C=O is an amide
thnk u yep ik 

Original post by RMNDK
You agree that a ketone functional group is this:

R must be either an alkyl group or aromatic group. In either case, you're going to have CARBON atoms bonded to the C=O.Wunderbarr is slightly mistaken.
It CANNOT be a Nitrogen or a Halogen or a Hydrogen or an Oxygen. If R was any of these, the functional group has changed; it's no longer a ketone.
If it's a Nitrogen, then it's an Amide/Amine
If it's a Halogen, then it's an Acyl Halide
If it's a Hydrogen, then it's an Aldehyde
If it's an Oxygen, then it's an Ester/Carboxylic Acid.
I hope you do know what those functional groups all are...

R must be either an alkyl group or aromatic group. In either case, you're going to have CARBON atoms bonded to the C=O.
Spoiler
It CANNOT be a Nitrogen or a Halogen or a Hydrogen or an Oxygen. If R was any of these, the functional group has changed; it's no longer a ketone.
If it's a Nitrogen, then it's an Amide/Amine
If it's a Halogen, then it's an Acyl Halide
If it's a Hydrogen, then it's an Aldehyde
If it's an Oxygen, then it's an Ester/Carboxylic Acid.
I hope you do know what those functional groups all are...
Quick Reply
Related discussions
- CHEM A-level HEL|P
- carbonyls a level chemistry
- A-Level chemistry
- propan-1-ol and propanal
- What type of isomerism do Ketones and Functional groups have?
- why reflux? (alcohol to ketone)
- Spectroscopy help
- Electrophillic/nucelophillic substitution /addition
- Functional Groups Revision
- Reaction of Alcohols
- How do you distinguish between primary and secondary alcohols by chemical reaction?
- Connectivity chem
- Chemistry
- A level chemistry optical isomerism MC questions
- is but-1-ene the same as butene
- Does anyone why the answer to this a level chemistry question is A
- what do the empty sticks mean in the chemistry data sheet?
- Chemistry A level
- A-Level chemistry
- Edexcel A-Level Chem Paper 2 Advanced Organic and Physical Chemistry [Exam Chat]
Latest
Trending
Last reply 4 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]Trending
Last reply 4 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]



