AQA AS chem multiple choice ques
How would you answer this? I don't really understand molecular ions
assuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
assuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
(edited 7 years ago)
Scroll to see replies
Original post by kiiten
How would you answer this?
I don't really understand molecular ionsassuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
I don't really understand molecular ionsassuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
Chlorine has 3 peaks.
35Cl-35Cl
35Cl-37Cl
37Cl-37Cl
Original post by TeachChemistry
Chlorine has 3 peaks.
35Cl-35Cl
35Cl-37Cl
37Cl-37Cl
35Cl-35Cl
35Cl-37Cl
37Cl-37Cl
what do you mean by Cl35 - Cl35
Original post by kiiten
what do you mean by Cl35 - Cl35
Cl has two isotopes. Cl 35 and Cl 37. The weighted mean mass gives 35.5 in the periodic table.
Original post by TeachChemistry
Cl has two isotopes. Cl 35 and Cl 37. The weighted mean mass gives 35.5 in the periodic table.
I know that but does that mean if there are 3 isotopes there will be 4 peaks or like chlorine 2 isotopes = 3 peaks?
Original post by kiiten
I know that but does that mean if there are 3 isotopes there will be 4 peaks or like chlorine 2 isotopes = 3 peaks?
With chlorine molecule there are three possibilities for the two atoms in the molecule.
They can comprise 2 35s, a 35 and a 37, or 2 37s. In other words you would see peaks at 70, 72 and 74.
Original post by kiiten
How would you answer this?
I don't really understand molecular ions
assuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
I don't really understand molecular ions
assuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
Molecular ion is just
Chlorine has two isotopes, Cl35 and Cl37; so in a compound containing one chlorine compound there's two molecular ions; M and M+2: one where the chlorine is Cl35 and one where it's Cl37. For dichloro- compounds you've got the M+4 where both chlorines are Cl37 in addition to the M and M+2. Likewise trichloro- compounds have an M+6 peak (all three the heavier isotope), and tetrachloro- compounds will have an M+8 peak in addition to the others. So C4H6Cl4 has five molecular ions. M; M+2; M+4; M+6 and M+8; giving five molecular ion peaks, although the intensities of the higher peaks will be a lot lower due to the relative abundance of Cl35 compared to Cl37.
Original post by Stiff Little Fingers
Molecular ion is just 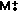 - it's the whole molecule - no fragmentation, just the loss of an electron.
- it's the whole molecule - no fragmentation, just the loss of an electron.
Chlorine has two isotopes, Cl35 and Cl37; so in a compound containing one chlorine compound there's two molecular ions; M and M+2: one where the chlorine is Cl35 and one where it's Cl37. For dichloro- compounds you've got the M+4 where both chlorines are Cl37 in addition to the M and M+2. Likewise trichloro- compounds have an M+6 peak (all three the heavier isotope), and tetrachloro- compounds will have an M+8 peak in addition to the others. So C4H6Cl4 has five molecular ions. M; M+2; M+4; M+6 and M+8; giving five molecular ion peaks, although the intensities of the higher peaks will be a lot lower due to the relative abundance of Cl35 compared to Cl37.
Chlorine has two isotopes, Cl35 and Cl37; so in a compound containing one chlorine compound there's two molecular ions; M and M+2: one where the chlorine is Cl35 and one where it's Cl37. For dichloro- compounds you've got the M+4 where both chlorines are Cl37 in addition to the M and M+2. Likewise trichloro- compounds have an M+6 peak (all three the heavier isotope), and tetrachloro- compounds will have an M+8 peak in addition to the others. So C4H6Cl4 has five molecular ions. M; M+2; M+4; M+6 and M+8; giving five molecular ion peaks, although the intensities of the higher peaks will be a lot lower due to the relative abundance of Cl35 compared to Cl37.
What about the carbon and hydrogen? I understand what you're saying but what do you mean by M+ and M+2 etc.

Original post by kiiten
What about the carbon and hydrogen? I understand what you're saying but what do you mean by M+ and M+2 etc. 

You're told to assume that carbon and hydrogen only have 1 isotope; so there's only one potential molecular weight for them.
M is the molecular ion; the mass of the molecule (assuming chlorine is present solely as Cl35) M+2 is that plus 2 mass units - assuming that one of the chlorines is Cl37 since Cl37 is two mass units heavier than Cl35. M+4 is the mass of the molecule plus 4 mass units, assuming 2 chlorines are the heavier isotope. M+6 is mass + 6 mass units for 3 of the heavier chlorine isotope, and so on.
Original post by Stiff Little Fingers
You're told to assume that carbon and hydrogen only have 1 isotope; so there's only one potential molecular weight for them.
M is the molecular ion; the mass of the molecule (assuming chlorine is present solely as Cl35) M+2 is that plus 2 mass units - assuming that one of the chlorines is Cl37 since Cl37 is two mass units heavier than Cl35. M+4 is the mass of the molecule plus 4 mass units, assuming 2 chlorines are the heavier isotope. M+6 is mass + 6 mass units for 3 of the heavier chlorine isotope, and so on.
M is the molecular ion; the mass of the molecule (assuming chlorine is present solely as Cl35) M+2 is that plus 2 mass units - assuming that one of the chlorines is Cl37 since Cl37 is two mass units heavier than Cl35. M+4 is the mass of the molecule plus 4 mass units, assuming 2 chlorines are the heavier isotope. M+6 is mass + 6 mass units for 3 of the heavier chlorine isotope, and so on.
so M+4 is Cl37 and Cl37 ? Back to the original ques, so the compound contains Cl4: M + M+2 and ??? I'm confused :'( I still don't understand
Original post by kiiten
so M+4 is Cl37 and Cl37 ? Back to the original ques, so the compound contains Cl4: M + M+2 and ??? I'm confused :'( I still don't understand
So M+4 for Chlorine would be Cl37 - Cl37 - both the heavier isotope, and the peak for it would at an m/z four more than the Cl35-Cl35 compound.
For your tetrachlorobutane, there's M; M+2; M+4; M+6 and M+8 - all the different potential combinations of Cl35 and Cl37.
Original post by Stiff Little Fingers
So M+4 for Chlorine would be Cl37 - Cl37 - both the heavier isotope, and the peak for it would at an m/z four more than the Cl35-Cl35 compound.
For your tetrachlorobutane, there's M; M+2; M+4; M+6 and M+8 - all the different potential combinations of Cl35 and Cl37.
For your tetrachlorobutane, there's M; M+2; M+4; M+6 and M+8 - all the different potential combinations of Cl35 and Cl37.
please could you write out the combinations like cl35 and cl37 because I don't understand M and M+2. sorry for causing you trouble
Original post by kiiten
please could you write out the combinations like cl35 and cl37 because I don't understand M and M+2. sorry for causing you trouble
Sure:
M - 4 x Cl35
M+2 - 3 x Cl35; 1 x Cl37
M+4 - 2 x Cl35; 2 x Cl37
M+6 - 1 x Cl35; 3 x Cl37
M+8 - 4 x Cl37
So M would be with the chlorines contributing 140 m.u. to the weight of the molecular ion. M+2 has chlorine contributing 142 m.u. thanks to the extra 2 m.u. from the heavier isotope. M+4 has the chlorines at 144 m.u., 2 extra m.u. from each of the chlorine, a total of 4 extra m.u. etc.
Original post by Stiff Little Fingers
Sure:
M - 4 x Cl35
M+2 - 3 x Cl35; 1 x Cl37
M+4 - 2 x Cl35; 2 x Cl37
M+6 - 1 x Cl35; 3 x Cl37
M+8 - 4 x Cl37
So M would be with the chlorines contributing 140 m.u. to the weight of the molecular ion. M+2 has chlorine contributing 142 m.u. thanks to the extra 2 m.u. from the heavier isotope. M+4 has the chlorines at 144 m.u., 2 extra m.u. from each of the chlorine, a total of 4 extra m.u. etc.
M - 4 x Cl35
M+2 - 3 x Cl35; 1 x Cl37
M+4 - 2 x Cl35; 2 x Cl37
M+6 - 1 x Cl35; 3 x Cl37
M+8 - 4 x Cl37
So M would be with the chlorines contributing 140 m.u. to the weight of the molecular ion. M+2 has chlorine contributing 142 m.u. thanks to the extra 2 m.u. from the heavier isotope. M+4 has the chlorines at 144 m.u., 2 extra m.u. from each of the chlorine, a total of 4 extra m.u. etc.
Thanks. so what would it be for Cl4 - I might ask my teacher to explain this to me as I don't get molecular ions as a whole
Original post by kiiten
Thanks. so what would it be for Cl4 - I might ask my teacher to explain this to me as I don't get molecular ions as a whole
You wouldn't have Cl4, that's not a compound you could form; chlorine gas is diatomic, so Cl2.
Posted from TSR Mobile
Original post by Stiff Little Fingers
You wouldn't have Cl4, that's not a compound you could form; chlorine gas is diatomic, so Cl2.
Posted from TSR Mobile
Posted from TSR Mobile
Sorry its C4H6Cl4
Posted from TSR Mobile
Original post by kiiten
That's tetrachlorobutane, which is the molecule I've been talking about in this thread. It's five peaks as below
Original post by Stiff Little Fingers
Chlorine has two isotopes, Cl35 and Cl37; so in a compound containing one chlorine compound there's two molecular ions; M and M+2: one where the chlorine is Cl35 and one where it's Cl37. For dichloro- compounds you've got the M+4 where both chlorines are Cl37 in addition to the M and M+2. Likewise trichloro- compounds have an M+6 peak (all three the heavier isotope), and tetrachloro- compounds will have an M+8 peak in addition to the others.
So C4H6Cl4 has five molecular ions. M; M+2; M+4; M+6 and M+8; giving five molecular ion peaks, although the intensities of the higher peaks will be a lot lower due to the relative abundance of Cl35 compared to Cl37.
Chlorine has two isotopes, Cl35 and Cl37; so in a compound containing one chlorine compound there's two molecular ions; M and M+2: one where the chlorine is Cl35 and one where it's Cl37. For dichloro- compounds you've got the M+4 where both chlorines are Cl37 in addition to the M and M+2. Likewise trichloro- compounds have an M+6 peak (all three the heavier isotope), and tetrachloro- compounds will have an M+8 peak in addition to the others.
So C4H6Cl4 has five molecular ions. M; M+2; M+4; M+6 and M+8; giving five molecular ion peaks, although the intensities of the higher peaks will be a lot lower due to the relative abundance of Cl35 compared to Cl37.
Original post by Stiff Little Fingers
That's tetrachlorobutane, which is the molecule I've been talking about in this thread. It's five peaks as below
How would you explain the original ques to a GCSE student (i think this would make it easier for me to understand). Thanks

Posted from TSR Mobile
Original post by kiiten
How would you explain the original ques to a GCSE student (i think this would make it easier for me to understand). Thanks 
Posted from TSR Mobile

Posted from TSR Mobile
assuming that chlorine exists as 2 isotopes, and that hydrogen and carbon exist as 1 isotope each, how many molecular ion peaks will be shown in the mass spectrum of C4H6Cl4?
The molecular ion is the one at m/z 194 - the molecular weight of C4H6Cl4 if the Cls were of mass 35. You're told that hydrogen and carbon are there as one isotope, so you can discount the effects of different isotopes of those into the peaks; and you're told that there are 2 isotopes of chlorine. So the simplified question you're actually answering is how many different combinations of Cl35 and Cl37 (the two chlorine isotopes) are possible?
chlorine has 2 isotopes. the number of molecular ion peaks in the mass spec of Cl2 is:
Again the molecular ion is the one at the m/z of Cl2 - there's two isotopes of chlorine, in how many ways could they combine in Cl2?
Original post by Stiff Little Fingers
The molecular ion is the one at m/z 194 - the molecular weight of C4H6Cl4 if the Cls were of mass 35. You're told that hydrogen and carbon are there as one isotope, so you can discount the effects of different isotopes of those into the peaks; and you're told that there are 2 isotopes of chlorine. So the simplified question you're actually answering is how many different combinations of Cl35 and Cl37 (the two chlorine isotopes) are possible?
Different combinations of the 2 isotopes in Cl4:
5? so:
4 x Cl35
4 x Cl37
3 x Cl35 and Cl37
3 x Cl37 and Cl35
2 x Cl35 and 2 x Cl37
I know that you discount carbon and hydrogen because they only exist as 1 - but why?
Again the molecular ion is the one at the m/z of Cl2 - there's two isotopes of chlorine, in how many ways could they combine in Cl2?
3? So Cl35 and Cl35
Cl37 and Cl35
Cl37 and Cl37
Posted from TSR Mobile
(edited 7 years ago)
Quick Reply
Related discussions
- A Level French Help
- A-level French Study Group 2022
- Wrong topic list for UCAS end of years mocks
- A level Spanish
- A level Spanish speaking
- A-Level French Study Group 2023-2024
- AQA A Level Law Paper 3 (7162/3) - 10th June 2024 [Exam Chat]
- La haine
- Advanced higher biology exam
- Retake A Level Maths in my 40s - Advice
- Exam board for science for a levels
- Retaking maths GCSE need desperate help!
- Could someone please give me some feedback on this Entre les murs essay
- Advice on which Med School to Apply
- AS Chemistry Paper 1 2022
- AQA A Level Spanish Paper 1 7692/1 - 7th June 2023 [Exam Chat]
- A Level French, AQA, June 2020, published as Nov 2020: Spain multigen families
- Study buddy for a levels
- GCSE Spanish
- aqa gcse spanish 150 word task
Latest
Last reply 1 minute ago
LSE anthropology and law 2024Last reply 2 minutes ago
Girl cries after first day of work "How do you have time for your life?"Chat
7
Last reply 7 minutes ago
Official University College London Applicant Thread for 2024Last reply 20 minutes ago
Is anyone going to graphic design in 2024 in university of Northampton?Posted 22 minutes ago
Online Delivery of Flowers to BangaloreLast reply 43 minutes ago
AQA A Level Sociology Paper 2 (7192/2) - 4th June 2024 [Exam Chat]Last reply 43 minutes ago
R u 18-25 & studying in a England Uni, spare a few mins to fill my survey. 1 wk left.Last reply 46 minutes ago
Edexcel A Level Economics A Paper 1 (9ECO 01) - 15th May 2024 [Exam Chat]Posted 53 minutes ago
st. andrews sixth form in cambridgeLast reply 1 hour ago
Anyone else applying/has applied to UCL's Robotics and AI MEng course??Last reply 1 hour ago
LSE International Social and Public Policy and Economics (LLK1) 2024 Thread



