Chemistry unit 6 June 2016
Scroll to see replies
Original post by samb1234
The mistake you made was the - sign. If you are doing it that way, i.e. reduced species -oxidised species, you do NOT change the sign of the ecell values, i.e. in this case you would have X--0.14
But why we don't change it, as in what they have put, it is the value for the reduction of Sn and it's oxidation should be the opposite of the equation with a change to the sign ?
Posted from TSR Mobile
Original post by PlayerBB
But why we don't change it, as in what they have put, it is the value for the reduction of Sn and it's oxidation should be the opposite of the equation with a change to the sign ?
Posted from TSR Mobile
Posted from TSR Mobile
There are different ways of approaching it. Either you flip the sign and treat it as a normal sum and add the two numbers together (my personal preference) or you can remember the formula Ecell = Ereduction -Eoxidation (which is doing exactly the same thing, Eoxidation is the reduction potential for the oxidised species so by subtracting it you are flipping the sign, just as you would if you did the first method)
Original post by samb1234
There are different ways of approaching it. Either you flip the sign and treat it as a normal sum and add the two numbers together (my personal preference) or you can remember the formula Ecell = Ereduction -Eoxidation (which is doing exactly the same thing, Eoxidation is the reduction potential for the oxidised species so by subtracting it you are flipping the sign, just as you would if you did the first method)
Ahh, sorry for asking, but if the question gave us that the reaction is feasible, and gave us one of the potentials, do we also do this or directly, go by Ecell= Ered - Eoxi ?
Posted from TSR Mobile
Original post by PlayerBB
Ahh, sorry for asking, but if the question gave us that the reaction is feasible, and gave us one of the potentials, do we also do this or directly, go by Ecell= Ered - Eoxi ?
Posted from TSR Mobile
Posted from TSR Mobile
It's the same process, the only difference with feasibility questions is you have to do what it tells you to do. Normally you would say the more negative one is the one which would be oxidised, but for feasibility you have to assume it happens as it tells you in the question and do the maths as if they are right and then comment after if it is feasible
Original post by samb1234
It's the same process, the only difference with feasibility questions is you have to do what it tells you to do. Normally you would say the more negative one is the one which would be oxidised, but for feasibility you have to assume it happens as it tells you in the question and do the maths as if they are right and then comment after if it is feasible
Ahhh, thank you SO MUCH!!!
Posted from TSR Mobile
Original post by Adorable98
http://qualifications.pearson.com/content/dam/pdf/A%20Level/Chemistry/2013/Exam%20materials/6CH08_01_que_20110119.pdf
Q2(ii)

The answer is:
The concentration of iodine is proportional to the titre
Why's that?
Q2(ii)
The answer is:
The concentration of iodine is proportional to the titre
Why's that?

Anyone ??

Original post by Adorable98
Anyone ?? 

This is what I wrote on my mock.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.

(edited 7 years ago)
Original post by Ayman!
This is what I wrote on my mock.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.
This is what I wrote on my mock.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.

So is that a formula I should know?

Original post by Ayman!
This is what I wrote on my mock.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.
This is what I wrote on my mock.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.

That NMR question was honestly one of the more difficult ones, I couldn't even understand the spectrum
Original post by demotivated
That NMR question was honestly one of the more difficult ones, I couldn't even understand the spectrum
Heck yeah. IAL May 15 and Jan 16 also have decent ones but not nearly as difficult.
Original post by Adorable98
So is that a formula I should know?

I think so. I'm not sure how to show that they're proportional otherwise. We learnt the C1V1 = C2V2 formula in GCSEs so it kinda just stuck.

Can anyone help me with an NMR question in Jan 2014 IAL https://52e1064b4dc7f9fd87a96faa7f7e5a9427a24547.googledrive.com/host/0B1ZiqBksUHNYNFpielJBNWdCRFU/January%202014%20(IAL)%20QP%20-%20Unit%206%20Edexcel%20Chemistry.pdf
Question 3 (h)
The answer for the displayed formula is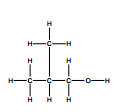
But I don't understand how to figure out that the methyl group is attached to a carbon from the spectrum? I would just draw 4 carbons in a straight chain
Question 3 (h)
The answer for the displayed formula is

But I don't understand how to figure out that the methyl group is attached to a carbon from the spectrum? I would just draw 4 carbons in a straight chain
Original post by demotivated
Can anyone help me with an NMR question in Jan 2014 IAL https://52e1064b4dc7f9fd87a96faa7f7e5a9427a24547.googledrive.com/host/0B1ZiqBksUHNYNFpielJBNWdCRFU/January%202014%20(IAL)%20QP%20-%20Unit%206%20Edexcel%20Chemistry.pdf
Question 3 (h)
The answer for the displayed formula is
But I don't understand how to figure out that the methyl group is attached to a carbon from the spectrum? I would just draw 4 carbons in a straight chain
Question 3 (h)
The answer for the displayed formula is

But I don't understand how to figure out that the methyl group is attached to a carbon from the spectrum? I would just draw 4 carbons in a straight chain
Check the spectrum. It shows that there are four different proton environments so based on the analysis of that spectrum, we can deduce a structure for the compound. If you see the spectrum, one of the environments shows around 8 small peaks and those number of peaks are actually telling us that the neighbouring carbon has 7 hydrogen around it which splits into 8(with reference to the n+1 rule).
I hope I'm correct lol I suck in this topic
Original post by Ayman!
Heck yeah. IAL May 15 and Jan 16 also have decent ones but not nearly as difficult.
I think so. I'm not sure how to show that they're proportional otherwise. We learnt the C1V1 = C2V2 formula in GCSEs so it kinda just stuck.
I think so. I'm not sure how to show that they're proportional otherwise. We learnt the C1V1 = C2V2 formula in GCSEs so it kinda just stuck.

Ayman, how are the moles of Iodine and Sodium Thiosulfate equal? Like did you assume this that they are equal?
Original post by sabahshahed294
Check the spectrum. It shows that there are four different proton environments so based on the analysis of that spectrum, we can deduce a structure for the compound. If you see the spectrum, one of the environments shows around 8 small peaks and those number of peaks are actually telling us that the neighbouring carbon has 7 hydrogen around it which splits into 8(with reference to the n+1 rule).
I think they are 9 peaks, but even then I don't see any carbon in the structure which has 8 hydrogen atoms attached to the carbons adjacent? It's also really inconvinient how we don't get the data booklet
Um, actually we should be provided with the data booklet because read what's written in page no 1.
Original post by Ayman!
Heck yeah. IAL May 15 and Jan 16 also have decent ones but not nearly as difficult.
I think so. I'm not sure how to show that they're proportional otherwise. We learnt the C1V1 = C2V2 formula in GCSEs so it kinda just stuck.
I think so. I'm not sure how to show that they're proportional otherwise. We learnt the C1V1 = C2V2 formula in GCSEs so it kinda just stuck.

I see, thank you!
Original post by Ayman!
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.
Has anyone done the NMR question on June 2013? It was one of those questions you'd really appreciate so long it didn't come in your exam.

Yeah, It was tough lol but tbh I found the question about electrode potentials in IAL January 2015 much harder..........If I have done this paper(Jan 2015), I would come out subbing from that question

Original post by Adorable98
Anyone ?? 

Also, in addition to what Ayman have wrote, Here as the volume of thiosulphate ions increase. then the concentration of Iodine increases as well that's because the concentration of iodine is directly proportional to the volume of thiosulphate ions
Which means, the more Sodium thiosulphate is added from the burette then it means there's more of the Iodine in the flask so the higher the concentration of Iodine
(edited 7 years ago)
Quick Reply
Related discussions
- GCSE Exam Discussions 2024
- A-level Exam Discussions 2024
- Edexcel Past Papers
- WJEC GCSE Biology Unit 1 Higher Tier [13th June 2023] Exam Chat
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- Edexcel A2 Biology Mind Maps For All Units/Topics
- btec applied science extended certificate aqa
- A Level Exam Discussions 2023
- Edexcel IAL Business Studies Notes
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- A Level Advice
- Biology rate of reaction question
- GCSE 2023 Grade Boundaries (All Exam Boards)
- oxfordAQA chemistry papers
- GCSE Biology Study Group 2022-2023
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Can I still get DDD with one Merit?
- do i do enough studying for a levels?
- Btec IT level 3 YR 2
Latest
Last reply 1 minute ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 minute ago
Official: University of St Andrews A100 Offer Holders 2024 entryPosted 2 minutes ago
MoJ Application status - what does 'on hold' mean?Last reply 5 minutes ago
Can I get an A/A* using claire bio and ms estruch vids for OCR A level biologyLast reply 13 minutes ago
Searching for a KCL Postgrad to take over my accommodation contractLast reply 13 minutes ago
TSR Study Together - STEM vs Humanities!Last reply 14 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 15 minutes ago
Weidenfeld Hoffmann Trust (WHT) Scholarship Notification (2024-2025)Last reply 15 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Last reply 16 minutes ago
yet to receive a woodhouse interviewLast reply 16 minutes ago
Government Social Research - Research Officer Scheme 2024Last reply 17 minutes ago
Official University of the Arts London Applicant Thread for 2024Trending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 6 days ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 6 days ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



