OCR Chemistry A - AS in Depth - 2015 (new spec) UNOFFICIAL MARK SCHEME
this is what I remember pls feel free to correct and also add missed questions.
the colour scheme is to try and separate whole questions.
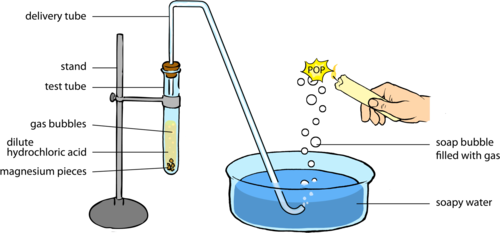
draw the apparatus you'd use:
I put a clamp stand with a syringe on the left, and a clamp stand holding a test tube with metal and water in with the bung connected to the syringe.
OR
the unknown metal: calcium
percentage yield: 67.4%
explain oxidation of magnesium in terms of electrons (1 mark)
oxidation of magnesium is loss of two electrons.
electron configuration of a magnesium atom
1s2 2s2 2p6 3s2
Magnesium vs Silicon
Magnesium = giant metallic, metallically bonded = electrostatic attraction between negatively charged delocalised sea of electrons and positively charged Mg ions.
Silicon = giant molecular, covalently bonded (electrostatic attraction between shared pairs of electron and the nuclei of bonding atoms), forming a lattice.
the difference in melting point - p4 and cl2 - on the graph chlorine was lower
I put: they are both covalently bonded, butchlorine is a smaller molecule, and so has weaker london forces therefore less energy is required to break the intermolecular forces therefore lower melting point.
second ionisation energy for strontium inc state symbols
Sr+ (g) --> Sr2+ (g) + e-
a question to do with explaining ionisation energy trend down group 2 but I can't remember btwn which elements
nuclear charge increases down the group however this is outweighed by increase in shielding and also increase in atomic radius, therefore there is less nuclear attraction (between nucleus and outer electrons) so outer electrons are more easily lost/less energy is required to remove them therefore lower first ionisation energy.
mass of strontium carbonate = 1.845g
rates question: why was there loss in mass or something?
(2 mol of) gas formed = products escaped.
describe and explain the trend in the graph up to 200s.
Steep negative gradient, mass rapidly decreases as time goes on = decreasing rate of reaction, reactants are being used up.
explain why enthalpy change of formation for NO was exothermic in terms of bonds
I put because more bonds made than were broken and making bonds releases energy whereas bond breaking uses up energy.
enthalpy profile diagram: endothermic
bottom left = reactants = N2 + 0.5 O2
top right = products = n20
pv=nrt question: (in standard form) 4.46*10^6 Pa
show NO catalyses the break down of ozone:NO• + O3 -> NO2• + O2NO2• + O -> NO• + O2
mechanism for the reaction of compound A an alkene with a hydrogen halide (HBr)
show the mechanism, the two different products, explain which of two would form more of.
unsymmetrical alkene CH3HC=CCH3CH3 , basically the second possible product just had the H and Br on the resulting haloalkane switched around, more of the tertiary halogenoalkane would be formed because it's more stable.
why can't compound A (alkene) exhibit E/Z isomerism?
because it doesn't have 2 different groups on each carbon of the double bond.
Z isomer for the alkene:
Z pent-2-ene
structural isomers: 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
why is it a secondary alcohol?
because the OH is attached to the carbon that is attached to 2 alkyl groups
write equation for butan-2-ol oxidising
I drew out: butan-2-ol + [O] - butanone + h20
bond angle around the oxygen in the butan-2-ol (3 marks)
104.5
reason: 2 bonding pairs and 2 lone pairs around the central atom, electron pairs repel to maximum separation = non-linear shape.
Spoiler
i didn’t know this but I put: because nowhere to separately collect the products, reflux apparatus would be better.
aluminium sulfide and water forming aluminium nitrate and hydrogen sulfide
Al2S3 + 6H2O -> 2Al(OH)3 + 3 H2S
last question (mass spectrum/ infrared red) conspiracy as it currently stands = methylpropanoic acid
Scroll to see replies
I got pent-2-ene for another
I don't know this but got 1.845
another question I got 0.48
percentage yield was 67% something, but I got 21.9 % how many marks could I get?
and the last one I got butanoic acid, but people are debating that it could be 2-methylpropanoic acid
I got pent-2-ene for another
I don't know this but got 1.845
another question I got 0.48
percentage yield was 67% something, but I got 21.9 % how many marks could I get?
and the last one I got butanoic acid, but people are debating that it could be 2-methylpropanoic acid
I got 1.845, I'm pretty sure it was the mass of SrCO3
I got pent-2-ene for another
I don't know this but got 1.845
another question I got 0.48
percentage yield was 67% something, but I got 21.9 % how many marks could I get?
and the last one I got butanoic acid, but people are debating that it could be 2-methylpropanoic acid
They said standard form so 4.46*10^6
1.845g was mass of SrCO3
For he last one I believe it was 2-methylpropanoic acid as it mentioned secondary carbonation
For percentage yield if you use the correct formula it should be at least 1/2 marks

Oxidising in terms of electrons - loses electrons (gives another species them)
magnesium - 1s2 2s2 2p6 3s2
pretty sure it was Sr+ (g) -> Sr2+ (g) + e-
How to set up the srco3 + hno3 expriment - i talked about weighing balance, and adding dilute nitric acid putting a bung on top into another test tube to collect the co2 and measure the mass of the srco3 every 50 or so seconds (pretty sure I got this wrong)
Something froming H2S was
Al2S3 + 6H2O -> 2Al(OH)3 + 3 H2S
Thats IS balanced (They didnt ask for a balanced equation anyway)
Edit: Oh crap, I put that then I crossed it out. brain fart
For the last question I got methyl propanoic acid
For the alkene --> alcohol question:
1.
No E/Z because C2 has the same group
2.
Z pent-2-ene was the name I got
3.
The alcohols I had were 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
P4 vs Cl2: P4 has more electrons so more London forces so greater attraction so more energy required to break bonds
1.845g was mass of SrCO3
For he last one I believe it was 2-methylpropanoic acid as it mentioned secondary carbonation
For percentage yield if you use the correct formula it should be at least 1/2 marks
did it have to be specific, could it not have been butanoic acid? I forget to include the secondary carbocation detail, but all the other evidences fit into this. maybe 5/6?
It could be butanoic acid but I think you have at least 5/6 if you said butanoic acid... The question said what is C not give a POSSIBLE structure of C

Oxidising in terms of electrons - loses electrons (gives another species them)
magnesium - 1s2 2s2 2p6 3s2
pretty sure it was Sr+ (g) -> Sr2+ (g) + e-
How to set up the srco3 + hno3 expriment - i talked about weighing balance, and adding dilute nitric acid putting a bung on top into another test tube to collect the co2 and measure the mass of the srco3 every 50 or so seconds (pretty sure I got this wrong)
for the drawing question, would u get a mark for drawing the clamp, and also on the outline the method question, I think u were meant to talk about the testing for ions, but I mentioned a weighing scale too
For the last question I got methyl propanoic acid
For the alkene --> alcohol question:
1.
No E/Z because C2 has the same group
2.
Z pent-2-ene was the name I got
3.
The alcohols I had were 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
P4 vs Cl2: P4 has more electrons so more London forces so greater attraction so more energy required to break bonds
That is balanced as 12H on RHS and 12 H on LHS
ahh tha good
Edit: Oh crap, I put that then I crossed it out. brain fart
For the last question I got methyl propanoic acid
For the alkene --> alcohol question:
1.
No E/Z because C2 has the same group
2.
Z pent-2-ene was the name I got
3.
The alcohols I had were 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
P4 vs Cl2: P4 has more electrons so more London forces so greater attraction so more energy required to break bonds
for the z isomer, could they possibly award all the marks if u missed out writing the z bit?
also, I talked about how p4 has more atoms bonded together, so more bonds are present in the molecule, which will require more energy to bread the molecule, whereas chlorine can only exist in diatomic so lower amount of energ7y is only required to break the bonds.
i did this lol :/ I hope they do....
"I talked about how p4 has more atoms bonded together, so more bonds are present in the molecule, which will require more energy to bread the molecule, whereas chlorine can only exist in diatomic so lower amount of energ7y is only required to break the bonds." yes this counts as basically saying p4 is a bigger molecule imo but you won't get all the marks until you mention london forces
also, I talked about how p4 has more atoms bonded together, so more bonds are present in the molecule, which will require more energy to bread the molecule, whereas chlorine can only exist in diatomic so lower amount of energ7y is only required to break the bonds.
Yeah they should award you as long as you mentioned pent-2-ene
They asked you to draw the Z isomer so you need that correct
I think you needed to have mentioned London forces for P4 and Cl2
Think I put 104.5
reason: 2 bonding pairs 2 lone pairs lone pairs repel bonding pairs more into a non linear shape.

Oxidising in terms of electrons - loses electrons (gives another species them)
magnesium - 1s2 2s2 2p6 3s2
pretty sure it was Sr+ (g) -> Sr2+ (g) + e-
How to set up the srco3 + hno3 expriment - i talked about weighing balance, and adding dilute nitric acid putting a bung on top into another test tube to collect the co2 and measure the mass of the srco3 every 50 or so seconds (pretty sure I got this wrong)
also that first diagram I swear they said they wanted to measure the volume that's why I put a syringe, originally I had put the image you've posted :/
I said really similar like
weigh the beaker, add the acid weigh again, weigh oxide and add it, start stopwatch as soon as you add the oxide
i didn't say specifically measure the mass of src03 because the graph was (regants + container)
except i said record reading on the scale every 10 seconds
I think I was wrong too though.
this is what I remember pls feel free to correct and also add.
the colour scheme is to try and separate whole questions.
draw the apparatus you'd use:
I made this one up - I put a clamp stand with a syringe on the left, and a clamp stand holding a test tube with magnesium and water in with a bong connected to the syringe.
the metal: calcium
percentage yield: 67.4%
Magnesium vs Silicon - really unsure on this one bc im crap at bonding
Magnesium = giant metallic, metallically bonded = electrostatic attraction between negatively charged delocalised sea of electrons and positively charged nuclei of bonding atoms.
Silicon = giant molecular (Covalent), electrostatic attraction between shared pairs of electron and the nuclei of bonding atoms, forming a lattice.
the difference in melting point - p4 and cl2 - on the graph chlorine was lower
I put: they are both covalently bonded, butchlorine is a smaller molecule, and so has weaker london forces therefore less energy is required to break the intermolecular forces therefore lower melting point.
second ionisation energy for strontium
Sr+ --> Sr2+ + e-
mass of strontium carbonate = 1.845g
rates question: why was there loss in mass or something?
I put cause the reactants were getting used up and (2 mol of gas given off)
describe and explain the trend in the graph up to 200s.
rate rapidly decreases.
(at least one of the reactants) is being used up (?)
explain why enthalpy change of formation for NO was exothermic in terms of bonds
I put because more bonds made than were broken and making bonds releases energy whereas bond breaking uses up energy.
show NO catalyses the break down of ozone:
NO• + O3 -> NO2• + O2
NO2• + O -> NO• + O2
enthalpy profile diagram: endothermic
bottom left = reactants = N2 + 0.5 O2
top right = products = n20
pv=nrt question: (in standard form) 4.46*10^6 Pa
mechanism for the reaction of compound A an alkene with a hydrogen halide (HBr)
show the mechanism, the two different products, explain which of two would form more of.
unsymmetrical alkene CH3HC=CCH3CH3 , basically the second possible product just had the H and Br on the resulting haloalkane switched around, more of the tertiary halogenoalkane would be formed because it's more stable.
why can't compound A (alkene) exhibit E/Z isomerism?
because it doesn't have 2 different groups on each carbon of the double bond.
Z isomer for the alkene:
Z pent-2-ene
structural isomers: 2 methyl 2 butan-2-ol and 3 methyl butan-2-ol
why is it a secondary carbocation?
because the halogen is attached to the carbon that is attached to 2 alkyl groups
write equation for butan-2-ol oxidising
I drew out: butan-2-ol + [O] - butanone + h20
why is this apparatus not suitable for oxidising a secondary alcohol?
i didn’t know this but I put: because nowhere to separately collect the products, reflux apparatus would be better.
aluminium sulfide and water forming aluminium nitrate and hydrogen sulfide
Al2S3 + 6H2O -> 2Al(OH)3 + 3 H2S
Can you please show some of the working out for the maths questions and say the full questions for the others, how many questions were there as well
Quick Reply
Related discussions
- OCR A biology (a level)
- AS Physics 2023 OCR A
- 1000+ A2-Level Biology Exam Questions
- How to get A* at OCR A level chemistry?
- OCR A-level Computer Science Paper 2 (H446/02) - 19th June 2023 [Exam Chat]
- AS/A Level Chemistry Study Group 2023/2024
- Revising for A level mocks
- Chemistry A-level
- URGENT!!!!!!!!!most effective way to study biology chemistry A level
- Failure
- Study Buddy
- Business Edexcel
- what website gives the content that's most accurate for OCR biology?
- OCR B A-level Physics Paper 1 Advancing Physics (H557/01) - 24th May 2023 [Exam Chat]
- Writing prompts in HK public exam
- Edexcel A-Level Chem Paper 1 Advanced Inorganic and Physical Chemistry [Exam Chat]
- How do I study for A Level physics ‘Explain’ questions?
- oxfordAQA chemistry papers
- OCR A LEVEL PHYSICS A paper 2 unofficial mark scheme
- GCSE Diary
Latest
Last reply 2 minutes ago
AQA A Level Sociology Paper 1 (7192/1) - 20th May 2024 [Exam Chat]Last reply 2 minutes ago
Can I do medicine with these gcse grades and what unis would be most likely to acceptLast reply 4 minutes ago
March 2024 Cambridge Mature Colleges Round - Anybody?Last reply 17 minutes ago
Official University College London Applicant Thread for 2024Last reply 20 minutes ago
LSE International Social and Public Policy and Economics (LLK1) 2024 ThreadLast reply 29 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 39 minutes ago
English Exam for Queen Mary University of LondonLast reply 42 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 46 minutes ago
Official Veterinary Medicine Applicants thread 2024 entryLast reply 50 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadTrending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



