Aqa chem 4/ chem 5 june 2016 thread
Scroll to see replies
Original post by Parallex
S and T both have 6 peaks. For S, there are 5 non-equivalent H peaks in the ring structure and 1 on the OH. For T, the same applies for the ring and the methyl group.
Oh! Got you. Thanks so much, that's really helped. Quite sneaky - I forgot that there's obviously another H atom bonded to the carbon which has the CH3 attached to it
Original post by Parallex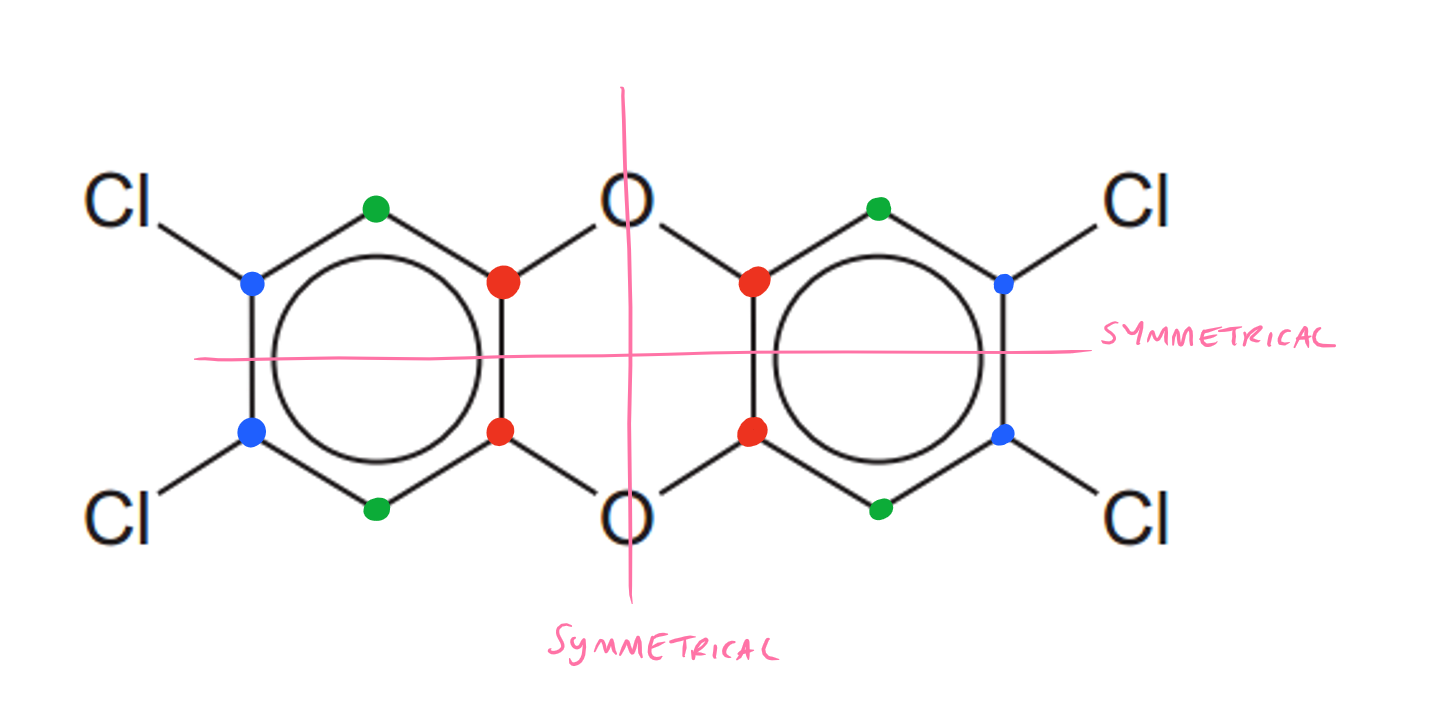
Equivalent carbon groups are the same colour. The compound is symmetrical about the O bonds so you only need to consider one half of the structure. Splitting the compound horizontally there is also a symmetry, so the groups below are equivalent to the groups above. There are 3 non-equivalent carbon groups in the molecule.

Equivalent carbon groups are the same colour. The compound is symmetrical about the O bonds so you only need to consider one half of the structure. Splitting the compound horizontally there is also a symmetry, so the groups below are equivalent to the groups above. There are 3 non-equivalent carbon groups in the molecule.
ah brilliant thanks.
Can you somehow explain 6bi to me, i've been trying to understand the ms for a while. I don't quite understand how the further substitutions work. Like what happened to the 3 hydrogens from the original ch3br
Original post by Super199
ah brilliant thanks.
Can you somehow explain 6bi to me, i've been trying to understand the ms for a while. I don't quite understand how the further substitutions work. Like what happened to the 3 hydrogens from the original ch3br
Can you somehow explain 6bi to me, i've been trying to understand the ms for a while. I don't quite understand how the further substitutions work. Like what happened to the 3 hydrogens from the original ch3br
Here's the mechanism so you can understand what's happening.
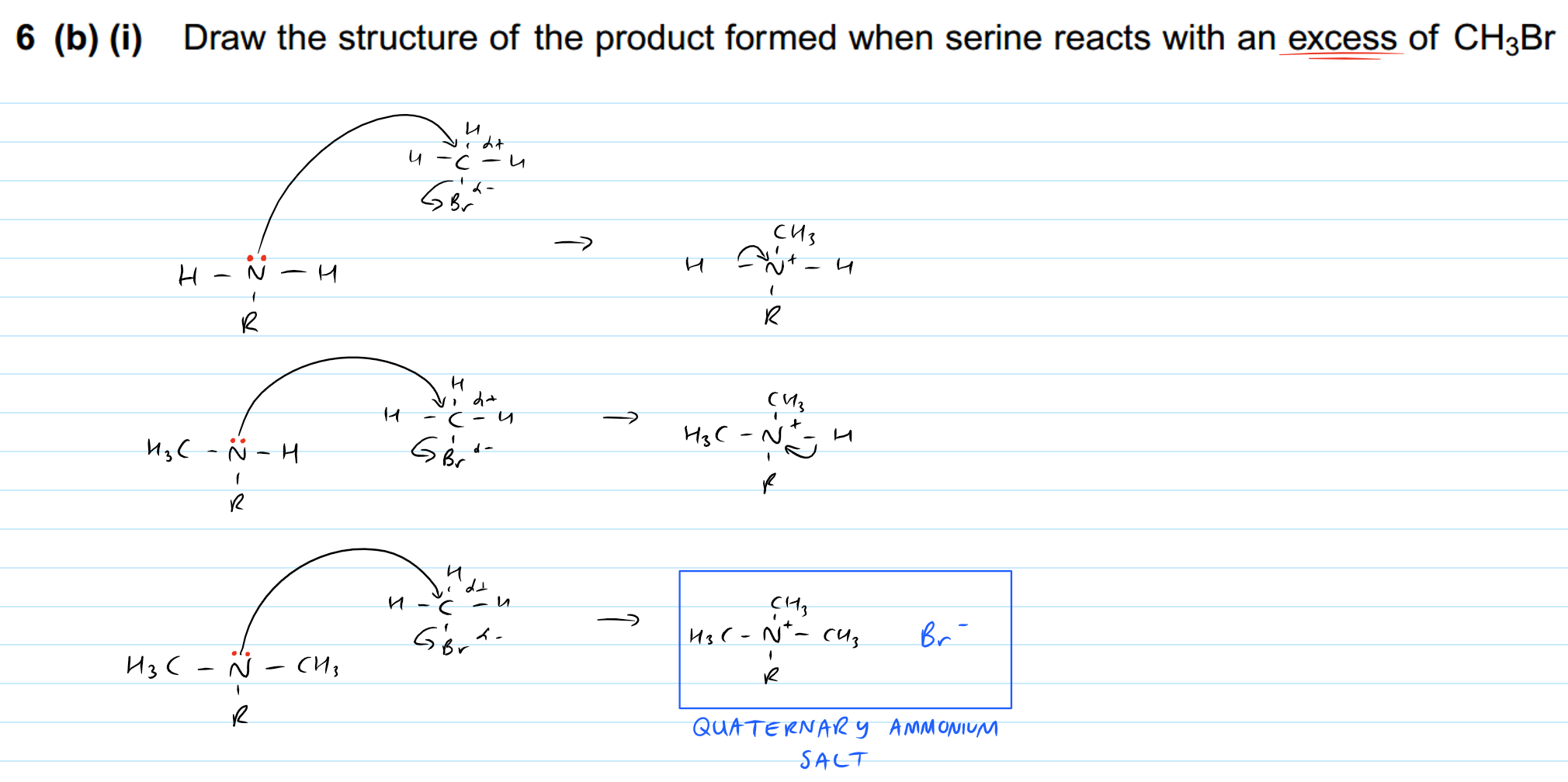
Notice that the lone pair remains on the N atom even after the first and second substitution. This means that it can still act as a nucleophile, so the same reaction that occurred in step 1 can occur again. In the 3rd substitution, the quaternary ammonium salt is formed and it isn't a nucleophile any longer. It bonds ionically with Br-.
Hi,
I don't understand how to qualitatively explain the effects of added OH-/ H+ to a buffer solution. Can somebody please explain this?
Thank you
I don't understand how to qualitatively explain the effects of added OH-/ H+ to a buffer solution. Can somebody please explain this?
Thank you
can anyone else just not get their head around any analytical??
Plane polarised
Original post by shiney101
Is it plane polarised or polarized light?
In a few of the recent papers polarized, whereas in the book and a 2012 paper it says polarised...
In a few of the recent papers polarized, whereas in the book and a 2012 paper it says polarised...
can someone help please?!
1e
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN14.PDF
1e
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN14.PDF
Original post by DiamondsForever
Hi,
I don't understand how to qualitatively explain the effects of added OH-/ H+ to a buffer solution. Can somebody please explain this?
Thank you
I don't understand how to qualitatively explain the effects of added OH-/ H+ to a buffer solution. Can somebody please explain this?
Thank you
HA disassociates Into H+ and A-
So if you add H+ = There is a large amount of A- in the buffer due to the salt - SO an increase in H+ will cause it to react with A- to form HA( equillbrium shifts to the left)
Adding OH- = OH- can react with H+ reducing the amount of H+ so equilibrium shifts to right
Can anyone please explain how to go about tackling this nmr type question i literally just dont no what to do 

Posted from TSR Mobile

Posted from TSR Mobile
Original post by Parallex
Only one of the indicators has pH 6 within its indicating range and that's 4-nitrophenol. At lower pH it is colourless and as pH increases it becomes yellow. The result is a mix between this and hence light yellow.
do you ignore the other indicators?
Original post by Superbubbles
do you ignore the other indicators?
It's not that you ignore them, it's that none of the other indicators have pH 6 within their indicating range.
HELP PLEASE!
Give an example of the use of a buffer solution.
Give an example of the use of a buffer solution.
Original post by Lilly1234567890
If Kc has increased, that means the forward reaction has been favoured (if Kc<1, reverse reaction is favoured, if Kc>1, forward reaction is favoured).
The forward reaction is exothermic (indicated by delta H<0).
Therefore, for the forward reaction to have been favoured (due to Kc having increased), the temperature must have decreased, as the equilibrium would shift to oppose the decrease in temperature by favouring the forward reaction.
Therefore, T1 is higher.
Original post by Lilly1234567890
HELP PLEASE!
Give an example of the use of a buffer solution.
Give an example of the use of a buffer solution.
Lactic acid and sodium lactate as an acidity regulator (appeared on June 13).
The ratio [HA]/[A-] remains fairly constant in the buffer. Any H+ added will react with A- to form HA and the equilibrium HA <------> A- + H+ will shift to oppose the change.
Original post by Sexybadman
Can anyone please explain how to go about tackling this nmr type question i literally just dont no what to do 

Posted from TSR Mobile

Posted from TSR Mobile
Integration value is the number of hydrogens for that particular peak, relative to the smallest non-zero number (which will usually be 1).
Use your data sheet to see what the peaks consist of - then start drawing. Trailing bonds on your data sheet will take the place of either hydrogens or other groups - use the integration value to deduce this.
Original post by Cadherin
Integration value is the number of hydrogens for that particular peak, relative to the smallest non-zero number (which will usually be 1).
Use your data sheet to see what the peaks consist of - then start drawing. Trailing bonds on your data sheet will take the place of either hydrogens or other groups - use the integration value to deduce this.
Use your data sheet to see what the peaks consist of - then start drawing. Trailing bonds on your data sheet will take the place of either hydrogens or other groups - use the integration value to deduce this.
Still struggling to understand what to do
 can you show maybe a worked example ?
can you show maybe a worked example ? 
Posted from TSR Mobile
Original post by Sexybadman
Still struggling to understand what to do  can you show maybe a worked example ?
can you show maybe a worked example ? 
Posted from TSR Mobile
 can you show maybe a worked example ?
can you show maybe a worked example ? 
Posted from TSR Mobile
What paper and which question is it? I c ant see it properly on my laptop
Quick Reply
Related discussions
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- GCSE Exam Discussions 2024
- TSR Study Together - STEM vs Humanities!
- A-level Exam Discussions 2024
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- reuben's y13/medapps journey!!
- A-level Biology Study Group 2022-2023
- Need Jan 2022 Past papers - Oxford AQA international A level BL05
- Grade Growth Chronicles | From C's to A's (23-24)
- Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05
- better late than never - gyg 24' :)
- GCSE Results: Post your results
- Holding myself accountable; study!
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- A Level Exam Discussions 2023
- C in Biology and D in Chem, while I resit could I do an intensive alevel in maths
- GYG a level y13⋆୨୧˚⟡˖ ࣪
- AS chemistry paper 2 2022 AQ
- AQA A Level Chemistry Paper 1 Practicals
- Biology Paper 1 - PRACTICE exam paper (with mark scheme) INVALUABLE resource
Latest
Last reply 3 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 5 minutes ago
student finance - mothers boyfriend taken into accountPosted 8 minutes ago
To Move Back to The UK and Work or Stay in Ireland and Go to Uni?Last reply 9 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 13 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadLast reply 18 minutes ago
Thales Degree Apprenticeship 2024Last reply 19 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 27 minutes ago
Can someone please mark my AIC essay (my teacher is such an unreliable marker)Last reply 34 minutes ago
Amazon Apprenticeships 2024Last reply 39 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 45 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2854
Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



