Aqa chem 4/ chem 5 june 2016 thread
Scroll to see replies
Original post by Sani Ej
Please can you explain q4a Jan -12 I really don't know where the answer came from
When you dilute an acid, use the formula
Same would apply for bases, replacing [H+] with [OH-]. (Click on the image to make it bigger btw.)
Original post by cutelady
But sometimes both species are present in two different reactions , so how would I know?
Posted from TSR Mobile
Posted from TSR Mobile
For the rate determining step look for whats in the rate equation and the number of mols of the speceis in the rate equation and if its the same as the rds.
Original post by Azzer11
Does anyone know why vdw forces act between terylene molecules instead of hydrogen bonding?
Posted from TSR Mobile
Posted from TSR Mobile
Thats a polyester right? I think for H-bonds, the electronegative atom has to be attached to a hydrogen. I remember reading something to that effect as to why carboxylic acids form H-bonds between themselves but ketones and aldehydes don't.
EDIT: Oops, didn't notice how many pages had passed since these questions were asked. Sorry if these questions have already been answered!
(edited 7 years ago)
Original post by hopingmedicinae
This is great but I lose it when is comes to 1/2 X moles of H+ neutralise 1/2x moles of OH-
would you be able to substitute some made up random simple numbers in it and exemplify?
Thankyou, sorry to be a pain!
would you be able to substitute some made up random simple numbers in it and exemplify?
Thankyou, sorry to be a pain!
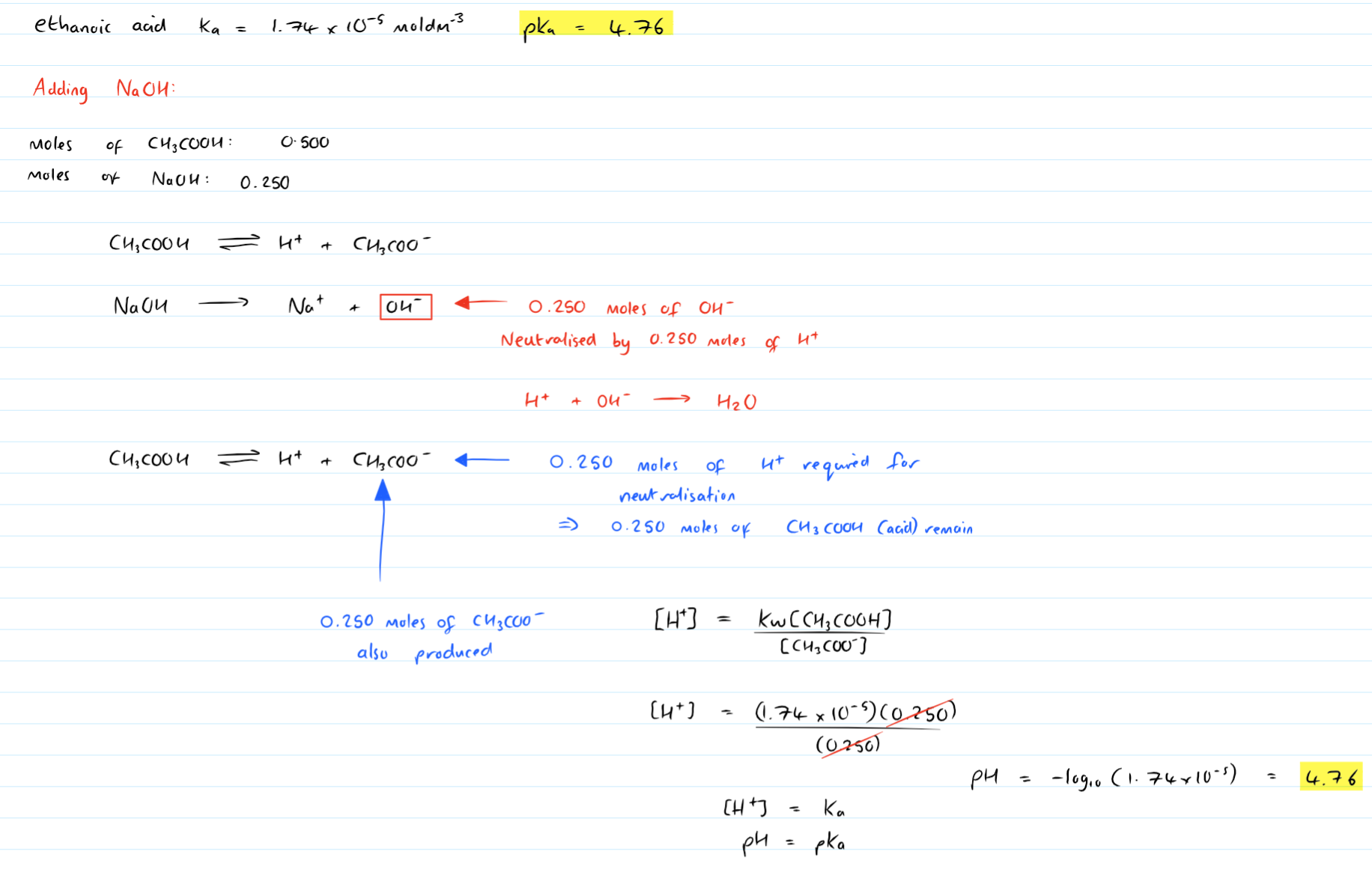
Original post by Super199
Alkenes react with aqueous bromine, alkanes don't.
CnH2n is either an alkene or cycloalkane.

Original post by Parallex
Alkenes react with aqueous bromine, alkanes don't.
CnH2n is either an alkene or cycloalkane.
CnH2n is either an alkene or cycloalkane.

Thanks

Do you mind quickly explaining the use of chromotography? and what q7b is on about?
What do we have to know about it?
Original post by Super199
Thanks 
Do you mind quickly explaining the use of chromotography? and what q7b is on about?
What do we have to know about it?

Do you mind quickly explaining the use of chromotography? and what q7b is on about?
What do we have to know about it?
Chromatography is a method of seperating similar compounds.
Alcohols are more polar than ketones due to the O-H bond being much more polar than the C=O bond. In column chromatography there is a stationary phase (silica) and the mixture of compounds is carried by the mobile phase which is a solvent. Each compound has a different affinity for the stationary phase. A greater affinity means it will take longer to reach the bottom of the column because there is a stronger attraction to the stationary phase. Propanol has the greater affinity for the stationary phase because it is more polar and hence a greater retention time, meaning propanone is the first to leave the column.
Original post by Parallex
Chromatography is a method of seperating similar compounds.
Alcohols are more polar than ketones due to the O-H bond being much more polar than the C=O bond. In column chromatography there is a stationary phase (silica) and the mixture of compounds is carried by the mobile phase which is a solvent. Each compound has a different affinity for the stationary phase. A greater affinity means it will take longer to reach the bottom of the column because there is a stronger attraction to the stationary phase. Propanol has the greater affinity for the stationary phase because it is more polar and hence a greater retention time, meaning propanone is the first to leave the column.
Alcohols are more polar than ketones due to the O-H bond being much more polar than the C=O bond. In column chromatography there is a stationary phase (silica) and the mixture of compounds is carried by the mobile phase which is a solvent. Each compound has a different affinity for the stationary phase. A greater affinity means it will take longer to reach the bottom of the column because there is a stronger attraction to the stationary phase. Propanol has the greater affinity for the stationary phase because it is more polar and hence a greater retention time, meaning propanone is the first to leave the column.
Do you mind listing the type of chromotography's and the stationary/liquid phases:
Is GLC: Stationary - liquid
mobile - inert gas
IDk what other types there are?
Edit: what does affinity mean sorry lol
Original post by Parallex
Chromatography is a method of seperating similar compounds.
Alcohols are more polar than ketones due to the O-H bond being much more polar than the C=O bond. In column chromatography there is a stationary phase (silica) and the mixture of compounds is carried by the mobile phase which is a solvent. Each compound has a different affinity for the stationary phase. A greater affinity means it will take longer to reach the bottom of the column because there is a stronger attraction to the stationary phase. Propanol has the greater affinity for the stationary phase because it is more polar and hence a greater retention time, meaning propanone is the first to leave the column.
Alcohols are more polar than ketones due to the O-H bond being much more polar than the C=O bond. In column chromatography there is a stationary phase (silica) and the mixture of compounds is carried by the mobile phase which is a solvent. Each compound has a different affinity for the stationary phase. A greater affinity means it will take longer to reach the bottom of the column because there is a stronger attraction to the stationary phase. Propanol has the greater affinity for the stationary phase because it is more polar and hence a greater retention time, meaning propanone is the first to leave the column.
Even though I didn't ask the question you definitely cleared up any confusion I had with chromatography so THANK YOU
 you explained this a lot better than my teacher
you explained this a lot better than my teacher 
Original post by Super199
Do you mind listing the type of chromotography's and the stationary/liquid phases:
Is GLC: Stationary - liquid
mobile - inert gas
IDk what other types there are?
Edit: what does affinity mean sorry lol
Is GLC: Stationary - liquid
mobile - inert gas
IDk what other types there are?
Edit: what does affinity mean sorry lol
Column:
Stationary - Silica gel.
Mobile - Solvent
GLC:
Stationary - powder coated with oil.
Mobile - unreactive gas (i.e. Nitrogen/Helium)
They're the only 2 we need to know I think.
Affinity in this case means the attraction to the stationary phase. Think of it like a magnet, the different compounds have a different affinity - or in this analogy - stronger and weaker attractions to the magnet. This leads to the compounds leaving the column at different times.
Original post by Parallex
Column:
Stationary - Silica gel.
Mobile - Solvent
GLC:
Stationary - powder coated with oil.
Mobile - unreactive gas (i.e. Nitrogen/Helium)
They're the only 2 we need to know I think.
Affinity in this case means the attraction to the stationary phase. Think of it like a magnet, the different compounds have a different affinity - or in this analogy - stronger and weaker attractions to the magnet. This leads to the compounds leaving the column at different times.
Stationary - Silica gel.
Mobile - Solvent
GLC:
Stationary - powder coated with oil.
Mobile - unreactive gas (i.e. Nitrogen/Helium)
They're the only 2 we need to know I think.
Affinity in this case means the attraction to the stationary phase. Think of it like a magnet, the different compounds have a different affinity - or in this analogy - stronger and weaker attractions to the magnet. This leads to the compounds leaving the column at different times.
What sort of qs do you normally get asked?
Original post by Super199
What sort of qs do you normally get asked?
It's usually just why one leaves the column first from the papers that I've done.
Original post by Parallex
For every 2mol of SO3 that breaks down, 2mol of SO2 and 1 mol of O2 are formed. We're not saying that some SO3 breaks down to form SO2 and some breaks down to O2, it's that each mole of SO3 that breaks down forms one mole of SO2 AND half a mole of O2.
Thank you so much, sorry I was having a very stupid moment
Original post by Parallex
It's usually just why one leaves the column first from the papers that I've done.
Which is just the solubility or what?
Original post by jessyoung
The 2015 paper was a joke
Yeaa can see this years being weird too :/
Posted from TSR Mobile
I AM GOING TO FAIL!!
Basically I am good with everything apart from when they ask you to deduce a structure of an isomer with the molecular formula "C6H12O2" i take too long on the questions, and I always end up getting them wrong! Does anyone have a quick method of working these out?
Posted from TSR Mobile
Basically I am good with everything apart from when they ask you to deduce a structure of an isomer with the molecular formula "C6H12O2" i take too long on the questions, and I always end up getting them wrong! Does anyone have a quick method of working these out?
Posted from TSR Mobile
Can someone explain to me why in q3bii we halve the values, when volume is doubled?
I know how to work out the answer but in terms of theory can someone explain it?
Thanks
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN14.PDF
I know how to work out the answer but in terms of theory can someone explain it?
Thanks
http://filestore.aqa.org.uk/subjects/AQA-CHEM4-QP-JUN14.PDF
Would someone mind listing the advtanges and diadvanted of incineration and different methods of diposal of polymers? All the one ive seen are somewhat wishy washy
Quick Reply
Related discussions
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- GCSE Exam Discussions 2024
- TSR Study Together - STEM vs Humanities!
- A-level Exam Discussions 2024
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- reuben's y13/medapps journey!!
- A-level Biology Study Group 2022-2023
- Need Jan 2022 Past papers - Oxford AQA international A level BL05
- Grade Growth Chronicles | From C's to A's (23-24)
- Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05
- better late than never - gyg 24' :)
- GCSE Results: Post your results
- Holding myself accountable; study!
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- A Level Exam Discussions 2023
- C in Biology and D in Chem, while I resit could I do an intensive alevel in maths
- GYG a level y13⋆୨୧˚⟡˖ ࣪
- AS chemistry paper 2 2022 AQ
- AQA A Level Chemistry Paper 1 Practicals
- Biology Paper 1 - PRACTICE exam paper (with mark scheme) INVALUABLE resource
Latest
Last reply 22 minutes ago
Official University College London Applicant Thread for 2024Last reply 27 minutes ago
Can I do economics degree without a level maths?Last reply 42 minutes ago
Official Cranfield University Applicant Thread for 2024Last reply 45 minutes ago
How to choose unis in UCAS application for CS undergraduate course?Last reply 57 minutes ago
Rishi Sunak pledges to remove benefits for people not taking jobs after 12 monthsLast reply 57 minutes ago
Why does he act different with me do I make him shy or what ?Last reply 1 hour ago
Got my crush's number 2 days ago and no reply yet. What could be the reasons?Posted 1 hour ago
GB News set to axe 40 jobs after channel posts heavy lossesLast reply 1 hour ago
Rwanda bill likely to be stalled at least till April after seven defeats in the LordsPosted 1 hour ago
Sunak rejects offer of youth mobility scheme between EU and UKLast reply 2 hours ago
Bank Of england degree apprenticeship 2024Trending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



