OCR Gateway Chemistry B - C1 C2 C3 - Unofficial Mark Scheme
I thought I might try and make a mark scheme for this as I haven't made one yet.
It might not be brilliant, but I think I can remember it reasonably well
It might not be brilliant, but I think I can remember it reasonably well

(edited 7 years ago)
Scroll to see replies
OCR Gateway Chemistry B
C1 C2 C3
Unofficial Mark Scheme
Friday 10 June 2016
If I have made any mistakes, feel free to correct me
I may have got some of the marks / order of the questions wrong
Module C1 - 25 marks
1) Carbon chemistry
a) Which is unsaturated? [1]
E (1)
b) Which have the same molecular formula? [1]
C and F (1)
c) What is the result of cracking C10H22 to form ethene? [1]
C8H18 (1)
d) Draw the displayed formula of poly(tetrafluoroethene) [1]
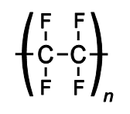 (1)
(1)
2) Solvents
a) Explain why the esters are not hydrocarbons [1]
do not only contain hydrogen and carbon / contain an oxygen atom in addition to hydrogen and carbon (1)
b) Predict the boiling point for the solvent [1]
range (about 150-170°) (1)
c) Evaluate its use as a solvent [2]
low melting point so liquid (1)
high boiling point so would not evaporate easily (1)
3) Carbon cycle and recycling of elements [QWC 6]
Marking points
why nitrogen stays constant: too unreactive - not used in carbon cycle
why carbon dioxide stays constant: removed by photosynthesis, but replaced by combustion and respiration
why oxygen stays constant: removed by respiration and combustion, but replaced by photosynthesis
effects of an increasing population: more deforestation, reducing plant numbers, and increasing carbon dioxide, and reducing oxygen; more burning of fossil fuels (see above); more respiration
4) Incomplete combustion of methane (olympic flame) to produce carbon [2]
CH4 + O2 = C + 2H2O
correct equation (2)
unbalanced but rest correct (1)
5) Paints
a) Graph for displaying the percentage solvent in each paint [1]
bar chart (1)
b) Graph for displaying [? can't remember] in each paint [1]
bar chart / pie chart (1)
c) Explain how an oil paint dries [2]
• the solvent evaporates (1)
• the oil is oxidised by atmospheric oxygen (1)
d) Paint is a colloid. Explain this [2]
particles mixed and dispersed in a liquid but not dissolved (1)
will not separate as the particles are too light (1)
6) GORE-TEX®
a) Explain how it works [2]
holes in membrane are too small for water topass through (1)
but are big enough for water vapourto pass through (1)
b) Suggest how it may be useful [1]
example: when hiking, the sweat can evaporate out and will not be uncomfortable (1)
there are other suitable examples that may be accepted as answers to this question
Module C2 - 25 marks
7) Alloys
a) Complete the table [3]
amalgam: mercury (1)
brass: copper and zinc (1)
solder: lead and tin (1)
b) Aluminium reaction with oxygen to make aluminium oxide - balanced symbol equation [2]
4Al + 3O2 -> 2Al2O3
correct equation (2)
unbalanced but rest correct (1)
8) Plane wings
a) Evaluate each material [3]
four correct evaluations (3)
three correct evaluations (2)
one or two correct evaluations (1)
b) Which material do you think is the best? [1]
A (1)
9) Explain why Wegener's theory is now accepted [2]
tested and discussed by many scientists (1)
explains a wide range of evidence, e.g. continents 'fitting' together (1)
10) Explain why the conditions used in the Haber process are not those that produce the maximum yield, using the graph [QWC 6]
will cover this later
11) Fertilisers
a) Why do fertilisers have to be soluble? [1]
plants absorb minerals through their roots (1)
b) Explain how eutrophication can occur [3]
algal bloom blocks off sunlight to other plants which die (1)
(aerobic) bacteria use up oxygen decaying these plants (1)
most living organisms die without this oxygen (1)
c) Word equation for reaction between potassium hydroxide and nitric acid [1]
potassium hydroxide + nitric acid -> potassium nitrate + water (1)
12) Rusting/corrosion
a) Explain how you know the material is aluminium [1]
does not corrode in dry or most air (1)
b) Word equation for rusting of iron [1]
iron + oxygen + water → hydrated iron(III) oxide (1)
c) Explain why the process is oxidation [1]
addition of oxygen / loss of electrons (1)
Module C3 - 25 marks
13) Reaction
a) Explain why the equation shows a conservation of mass [2]
calculation of relative formula masses for each side of the equation (1)
idea that they are equal and so mass is conserved (1)
b) Balanced symbol equation [1]
I can't remember this yet
14) Calculations
a) Show the predicted mass [1]
use of calculations correctly (1)
b) Calculation of percentage yield [2]
I can't remember this yet
correct answer (2)
incorrect answer with working (1)
15) Chromatography and medicine
a) Explain how the drug is extracted from the plant leaves, and how the tests determine its purity [QWC 6]
Marking points
how the drug is extracted: crushing to release the chemical; boiling and dissolving in suitable solvent; chromatography to separate out the chemical
what the tests show: chromatography shows there is an impurity as there are two dots; melting points show that there is an impurity as the melting points are below the pure chemical value, and there is a range - however they are close meaning that it is still reasonably pure
b) Explain why it is difficult to test and develop new pharmaceutical drugs that are safe to use [2]
any two of
labour costs (1)
difference between animal and human biology (1)
animal testing being unethical (1)
tests on cells are not always conclusive (1)
16) Calculations
a) Calculate the energy released [1]
32000J (1)
b) Calculate the temperature change [3]
32000/(4.2*100) = 76.1904762°C
76.2°C (3)
incorrect answer with all workings listed (2)
incorrect answer with some workings listed (1)
17) Explain why combustion is exothermic in terms of bond breaking and bond making [3]
more bonds made than bonds broken (1)
bond breaking releases energy (1)
therefore exothermic as more energy given out than taken in (1)
18) Reaction
a) Calculate the rate of reaction [1]
can't remember but it might have been around this
1.47 (1)
b) can't remember this [1]
c) Explain why increasing the concentration increases collision frequency [2]
more particles in a given area (1)
more likely to collide and react (1)
C1 C2 C3
Unofficial Mark Scheme
Friday 10 June 2016
If I have made any mistakes, feel free to correct me

I may have got some of the marks / order of the questions wrong
Module C1 - 25 marks
1) Carbon chemistry
a) Which is unsaturated? [1]
E (1)
b) Which have the same molecular formula? [1]
C and F (1)
c) What is the result of cracking C10H22 to form ethene? [1]
C8H18 (1)
d) Draw the displayed formula of poly(tetrafluoroethene) [1]
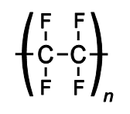 (1)
(1)2) Solvents
a) Explain why the esters are not hydrocarbons [1]
do not only contain hydrogen and carbon / contain an oxygen atom in addition to hydrogen and carbon (1)
b) Predict the boiling point for the solvent [1]
range (about 150-170°) (1)
c) Evaluate its use as a solvent [2]
low melting point so liquid (1)
high boiling point so would not evaporate easily (1)
3) Carbon cycle and recycling of elements [QWC 6]
Marking points
why nitrogen stays constant: too unreactive - not used in carbon cycle
why carbon dioxide stays constant: removed by photosynthesis, but replaced by combustion and respiration
why oxygen stays constant: removed by respiration and combustion, but replaced by photosynthesis
effects of an increasing population: more deforestation, reducing plant numbers, and increasing carbon dioxide, and reducing oxygen; more burning of fossil fuels (see above); more respiration
4) Incomplete combustion of methane (olympic flame) to produce carbon [2]
CH4 + O2 = C + 2H2O
correct equation (2)
unbalanced but rest correct (1)
5) Paints
a) Graph for displaying the percentage solvent in each paint [1]
bar chart (1)
b) Graph for displaying [? can't remember] in each paint [1]
bar chart / pie chart (1)
c) Explain how an oil paint dries [2]
• the solvent evaporates (1)
• the oil is oxidised by atmospheric oxygen (1)
d) Paint is a colloid. Explain this [2]
particles mixed and dispersed in a liquid but not dissolved (1)
will not separate as the particles are too light (1)
6) GORE-TEX®
a) Explain how it works [2]
holes in membrane are too small for water topass through (1)
but are big enough for water vapourto pass through (1)
b) Suggest how it may be useful [1]
example: when hiking, the sweat can evaporate out and will not be uncomfortable (1)
there are other suitable examples that may be accepted as answers to this question
Module C2 - 25 marks
7) Alloys
a) Complete the table [3]
amalgam: mercury (1)
brass: copper and zinc (1)
solder: lead and tin (1)
b) Aluminium reaction with oxygen to make aluminium oxide - balanced symbol equation [2]
4Al + 3O2 -> 2Al2O3
correct equation (2)
unbalanced but rest correct (1)
8) Plane wings
a) Evaluate each material [3]
four correct evaluations (3)
three correct evaluations (2)
one or two correct evaluations (1)
b) Which material do you think is the best? [1]
A (1)
9) Explain why Wegener's theory is now accepted [2]
tested and discussed by many scientists (1)
explains a wide range of evidence, e.g. continents 'fitting' together (1)
10) Explain why the conditions used in the Haber process are not those that produce the maximum yield, using the graph [QWC 6]
will cover this later
11) Fertilisers
a) Why do fertilisers have to be soluble? [1]
plants absorb minerals through their roots (1)
b) Explain how eutrophication can occur [3]
algal bloom blocks off sunlight to other plants which die (1)
(aerobic) bacteria use up oxygen decaying these plants (1)
most living organisms die without this oxygen (1)
c) Word equation for reaction between potassium hydroxide and nitric acid [1]
potassium hydroxide + nitric acid -> potassium nitrate + water (1)
12) Rusting/corrosion
a) Explain how you know the material is aluminium [1]
does not corrode in dry or most air (1)
b) Word equation for rusting of iron [1]
iron + oxygen + water → hydrated iron(III) oxide (1)
c) Explain why the process is oxidation [1]
addition of oxygen / loss of electrons (1)
Module C3 - 25 marks
13) Reaction
a) Explain why the equation shows a conservation of mass [2]
calculation of relative formula masses for each side of the equation (1)
idea that they are equal and so mass is conserved (1)
b) Balanced symbol equation [1]
I can't remember this yet
14) Calculations
a) Show the predicted mass [1]
use of calculations correctly (1)
b) Calculation of percentage yield [2]
I can't remember this yet
correct answer (2)
incorrect answer with working (1)
15) Chromatography and medicine
a) Explain how the drug is extracted from the plant leaves, and how the tests determine its purity [QWC 6]
Marking points
how the drug is extracted: crushing to release the chemical; boiling and dissolving in suitable solvent; chromatography to separate out the chemical
what the tests show: chromatography shows there is an impurity as there are two dots; melting points show that there is an impurity as the melting points are below the pure chemical value, and there is a range - however they are close meaning that it is still reasonably pure
b) Explain why it is difficult to test and develop new pharmaceutical drugs that are safe to use [2]
any two of
labour costs (1)
difference between animal and human biology (1)
animal testing being unethical (1)
tests on cells are not always conclusive (1)
16) Calculations
a) Calculate the energy released [1]
32000J (1)
b) Calculate the temperature change [3]
32000/(4.2*100) = 76.1904762°C
76.2°C (3)
incorrect answer with all workings listed (2)
incorrect answer with some workings listed (1)
17) Explain why combustion is exothermic in terms of bond breaking and bond making [3]
more bonds made than bonds broken (1)
bond breaking releases energy (1)
therefore exothermic as more energy given out than taken in (1)
18) Reaction
a) Calculate the rate of reaction [1]
can't remember but it might have been around this
1.47 (1)
b) can't remember this [1]
c) Explain why increasing the concentration increases collision frequency [2]
more particles in a given area (1)
more likely to collide and react (1)
(edited 7 years ago)
I haven't made it but will make it bit by bit 

Wow !! How do u remember this😂😂
Posted from TSR Mobile
Posted from TSR Mobile
Original post by Tea2345
Not too sure

Original post by some-student
Not too sure 

well I'm sure if u are able to memorise all these tests u will have smashed ur exams !! Have u finished all of yours yet?
Original post by Tea2345
well I'm sure if u are able to memorise all these tests u will have smashed ur exams !! Have u finished all of yours yet?
Nearly - I only have further maths left for Friday and then I am done

How about you?
I'm not sure how well I will have done overall but thanks

Original post by lovelybow
Well, you've got really good memory, wow! Amazing results await you on results day!! 

Thanks
 but I'm sure you'll do brilliantly as well
but I'm sure you'll do brilliantly as wellOriginal post by CandC
For the graph questions I did bar chart then pie chart. (Still unsure about this answer because everyone got very different but mainly in my school this was the answer) The question about aeroplanes, the desired metal was A. Just have to say again, master of mark schemes 🙌
Haha thank you

For the graphs, most people I talked to thought it was a bar chart, so I might but both as it was very confusing.
And I'll update the desired metal to be A...

Original post by Jiff
Thanks for this! What do you think the predicted grade boundaries will be? 

Thanks

I'm not sure about the grade boundaries, but they will probably be similar to or a little higher than 2015's grade boundaries.
2015: A* - 61, A - 51, B - 41, C - 31
My predictions for 2016 (might be completely wrong):
A* - 63, A - 54, B - 43, C - 35
Original post by some-student
Nearly - I only have further maths left for Friday and then I am done 
How about you?
I'm not sure how well I will have done overall but thanks

How about you?
I'm not sure how well I will have done overall but thanks

Yeah I have maths tomorrow too !! Bjt nervous as I made a couple of really stupid mistakes on Monday & really want an A* (A^ is unrealistic for me lol) what grade are you aiming for ?
Posted from TSR Mobile
Original post by Tea2345
Yeah I have maths tomorrow too !! Bjt nervous as I made a couple of really stupid mistakes on Monday & really want an A* (A^ is unrealistic for me lol) what grade are you aiming for ?
Posted from TSR Mobile
Posted from TSR Mobile
I'd like an A^ ideally (but would not be disappointed by any means with an A*) because I'm taking Maths and Further Maths A-level.
I think the exam on Monday went OK but I feel I made quite a lot of silly mistakes in the exam. Good luck for tomorrow

Original post by some-student
I'd like an A^ ideally (but would not be disappointed by any means with an A*) because I'm taking Maths and Further Maths A-level.
I think the exam on Monday went OK but I feel I made quite a lot of silly mistakes in the exam. Good luck for tomorrow
I think the exam on Monday went OK but I feel I made quite a lot of silly mistakes in the exam. Good luck for tomorrow

Aagh I'm too much of a wimp to take further maths at A level even though my teacher said I could😬😬 I'm doing bio though which is really content heavy so thought I'd stick to 4 at AS haha!!
Thanks ! Good luck to you aswell xx
Posted from TSR Mobile
Original post by some-student
Nearly - I only have further maths left for Friday and then I am done 
How about you?
I'm not sure how well I will have done overall but thanks

How about you?
I'm not sure how well I will have done overall but thanks

Thanks for the boundaries! Also I'm doing further maths too, I found the first one okay but I hope this one will be much better. Good luck

Original post by Jiff
Thanks for the boundaries! Also I'm doing further maths too, I found the first one okay but I hope this one will be much better. Good luck 

Same - good luck to you too

Original post by some-student
I thought I might try and make a mark scheme for this as I haven't made one yet.
It might not be brilliant, but I think I can remember it reasonably well
It might not be brilliant, but I think I can remember it reasonably well

Hi i was just wondering this is the ocr c1,2,3 chemistry b paper for june 2016 right?
So is the haber process wrong for ocr chemistry b gcse then??
(edited 3 years ago)
Quick Reply
Related discussions
- best way to revise maths
- Arsey's solutions
- Maths for economists - constrained maximisation questoin
- Isaac physics SPC 2022
- Edexcel A2 Mathematics: Core Mathematics C4 6666 01 - 22 June 2018 [Exam Discussion]
- Senior Physics Challenge 2022
- Physics Question help
- Ln integration
- Senior Physics Challenge Isaac Physics
- Isaac Senior Physics Challenge 2024
- OCR A-Level Chemistry B Paper 2 (H433/02) - 19th June 2023 [Exam Chat]
- I am looking at my GCSE grades from 2017
- GCSE Results: Post your results
- A Level Physics OCR A Paper 2 unofficial mark scheme
- What websites are good for learning GCSE Physics?
- OCR A LEVEL PHYSICS A paper 2 unofficial mark scheme
- Edexcel Past Papers
- How to revise for a level chemistry
- AS Physics 2023 OCR A
- Physics
Latest
Last reply 9 minutes ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 24 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 hour ago
Official University of Edinburgh Applicant Thread for 2024Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



