Finding Electrode Potential of an electrolytic cell
I'm facing problem with part(b) of this question:
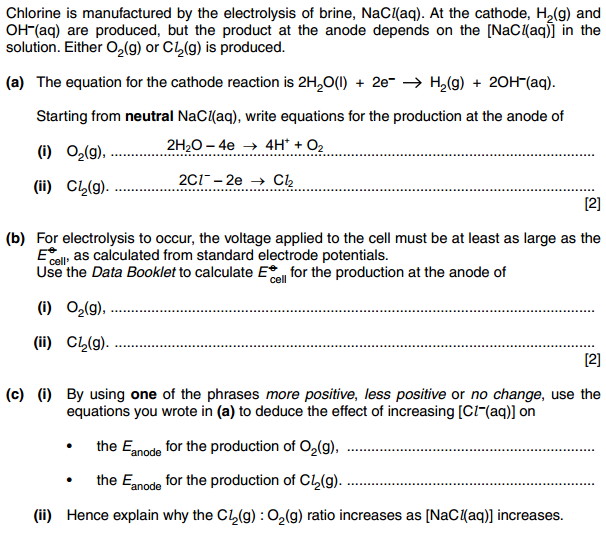
Here are the Electrode potential values of half cells that are being used:
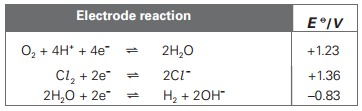
There are many ways of finding the Electrode potential of an electrochemical cell, by using different formulas. But I prefer to add up the electrode potentials of two half cells after reversing the sign of the electrode potential of the half cell that is shifting towards the left hand side of the equation. The equilibrium with more positive E naught value shifts to the right and and less positive(or more negative) one shifts to the left.
So if I apply the same concept here, the reactions are occurring in opposite direction to what E naught values predict, so I get negative Cell electrode potential values. Mark scheme just subtracts the electrode potential value of reaction at cathode from electrode potential values of reactions at anode.
Where am I going wrong?

Here are the Electrode potential values of half cells that are being used:

There are many ways of finding the Electrode potential of an electrochemical cell, by using different formulas. But I prefer to add up the electrode potentials of two half cells after reversing the sign of the electrode potential of the half cell that is shifting towards the left hand side of the equation. The equilibrium with more positive E naught value shifts to the right and and less positive(or more negative) one shifts to the left.
So if I apply the same concept here, the reactions are occurring in opposite direction to what E naught values predict, so I get negative Cell electrode potential values. Mark scheme just subtracts the electrode potential value of reaction at cathode from electrode potential values of reactions at anode.
Where am I going wrong?
(edited 11 years ago)
Original post by Zishi
I'm facing problem with part(b) of this question:

Here are the Electrode potential values of half cells that are being used:

There are many ways of finding the Electrode potential of an electrochemical cell, by using different formulas. But I prefer to add up the electrode potentials of two half cells after reversing the sign of the electrode potential of the half cell that is shifting towards the left hand side of the equation. The equilibrium with more positive E naught value shifts to the right and and less positive(or more negative) one shifts to the left.
So if I apply the same concept here, the reactions are occurring in opposite direction to what E naught values predict, so I get negative Cell electrode potential values. Mark scheme just subtracts the electrode potential value of reaction at cathode from electrode potential values of reactions at anode.
Where am I going wrong?

Here are the Electrode potential values of half cells that are being used:

There are many ways of finding the Electrode potential of an electrochemical cell, by using different formulas. But I prefer to add up the electrode potentials of two half cells after reversing the sign of the electrode potential of the half cell that is shifting towards the left hand side of the equation. The equilibrium with more positive E naught value shifts to the right and and less positive(or more negative) one shifts to the left.
So if I apply the same concept here, the reactions are occurring in opposite direction to what E naught values predict, so I get negative Cell electrode potential values. Mark scheme just subtracts the electrode potential value of reaction at cathode from electrode potential values of reactions at anode.
Where am I going wrong?
the way i do it which is right is, always take the more negative value, flip it, and add it to the other value.
so if u have potentials of -0.8 and -0.3, the more negative one is 0.8, so flip it, and add it to the other value and ull get 0.5. that is the spontaneous reaction. if u dont get a positive value when u do this, then it wouldn't be spontaneous.

Original post by aeyurttaser13
the way i do it which is right is, always take the more negative value, flip it, and add it to the other value.
so if u have potentials of -0.8 and -0.3, the more negative one is 0.8, so flip it, and add it to the other value and ull get 0.5. that is the spontaneous reaction. if u dont get a positive value when u do this, then it wouldn't be spontaneous.
so if u have potentials of -0.8 and -0.3, the more negative one is 0.8, so flip it, and add it to the other value and ull get 0.5. that is the spontaneous reaction. if u dont get a positive value when u do this, then it wouldn't be spontaneous.

I would like to get a second opinion on it.
BUMP!
My electrochemistry is so rusty I wouldn't put too much faith on me being right here  I'd rather drink off milk than do electrochem...
I'd rather drink off milk than do electrochem...
Ecell = E(more positive) - E(less positive) (NB when they're written as reductions)
Also in question a you never write negative quantities in chemical equations so should be 2H2O ---> 4H+ + O2 + 4e- instead
 I'd rather drink off milk than do electrochem...
I'd rather drink off milk than do electrochem...Ecell = E(more positive) - E(less positive) (NB when they're written as reductions)
Also in question a you never write negative quantities in chemical equations so should be 2H2O ---> 4H+ + O2 + 4e- instead

Original post by EierVonSatan
My electrochemistry is so rusty I wouldn't put too much faith on me being right here  I'd rather drink off milk than do electrochem...
I'd rather drink off milk than do electrochem...
Ecell = E(more positive) - E(less positive) (NB when they're written as reductions)
Also in question a you never write negative quantities in chemical equations so should be 2H2O ---> 4H+ + O2 + 4e- instead
 I'd rather drink off milk than do electrochem...
I'd rather drink off milk than do electrochem...Ecell = E(more positive) - E(less positive) (NB when they're written as reductions)
Also in question a you never write negative quantities in chemical equations so should be 2H2O ---> 4H+ + O2 + 4e- instead

Ah, right. So the "equilibrium with more positive Eo value will move to right and the equilibrium with less positive Eo will move to left" rule won't apply here because that's an electrolytic cell, not an electrochemical cell, right?
Original post by Zishi
Ah, right. So the "equilibrium with more positive Eo value will move to right and the equilibrium with less positive Eo will move to left" rule won't apply here because that's an electrolytic cell, not an electrochemical cell, right?
Moving right/left means nothing to me, sorry

It's probably better you stick to the way you've been taught to do this...
Original post by EierVonSatan
Moving right/left means nothing to me, sorry 
It's probably better you stick to the way you've been taught to do this...

It's probably better you stick to the way you've been taught to do this...
Ahh, no problem. Thanks anyway.
I hope charco will help.
charco
Quick Reply
Related discussions
- Question
- Electrochemistry help
- Electrochemical cell diagram: when do you add H+ to the solution? a level chem
- Chem a level - electrochemistry
- A level chemistry ocr
- Need Help on an Electrochem Q
- Electrochemistry
- Electrode potentials and equilibria?
- AQA A Level Chemistry Electrochemistry
- Electrochemical cells AQA A Level chemistry
- AQA A Level Chemistry Electrochemical Cells
- A level physics help
- Urgent OCR Chemistry Help
- A-level Chemistry Study Group 2022-2023
- Chemistry
- A&P revision recommendations
- TSR Study Together - STEM vs Humanities - Seventh Session
- Edexcel GCSE Chemistry Paper 1 Higher Tier 1CH0 1H - 27 May 2022 [Exam Chat]
- AQA A Level Chemistry Paper 3 7405/3 - 23 Jun 2022 [Exam Chat]
- Chemistry a level ocr
Latest
Last reply 1 minute ago
HSBC Degree Apprenticeship 2024Last reply 1 minute ago
Amazon Project management apprenticeship 2024Posted 2 minutes ago
Rishi Sunak pledges to remove benefits for people not taking jobs after 12 monthsLast reply 2 minutes ago
CPS Pupillage/Legal Trainee Scheme 2024 (2025 Start)Last reply 3 minutes ago
Official University of St Andrews Applicant Thread for 2024Last reply 5 minutes ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 5 minutes ago
emmanuella's study discussions #2: taking breaksLast reply 6 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 6 minutes ago
Official: King's College London A100 2024 Entry ApplicantsMedical Schools
1016
Last reply 8 minutes ago
Official University College London Applicant Thread for 2024Last reply 10 minutes ago
Plymouth university-transfer from biomedical science to dentistry


