This discussion is now closed.
Check out other Related discussions
- Should I apply to Cambridge?
- EPQ ideas - Physics
- A level mechanics help
- Moments
- Astrophysics
- Mechanics A Level Maths Question
- Trigonometric Modelling
- Moles in A-Level Chemistry
- As mechanics question
- Physics personal statement advice
- AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 25th May 2023 [Exam Chat]
- as level mechanics suvat
- Need help with kinematic question (AS)
- Uni physics advice
- ladders!!! - M2
- GCSE AQA Physics Paper 1 and 2 Revision and Study Chat
- Can someone help me understand these definitions in mechanics?
- Physics or Computer science?
- m1 june 2002 paper
- Gcse physics mocks
Applications of Particle in a Box model (Chemistry)
Scroll to see replies
Lifeisnice
What physical reality does n=2 represent
By itself? Nothing
and what does it mean when compared against n=3?
Energy of the particle is greater when n = 3 compared to n = 2, the probability of finding a particle at any one place is altered.
Lifeisnice
What physical reality does n=2 represent, and what does it mean when compared against n=3?
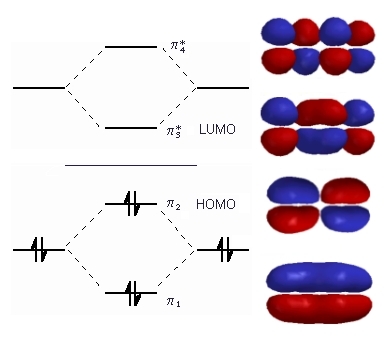
This image shows the states corresponding to n=1, 2, 3 and 4 for butadiene. As you can see, each state can be occupied by 2 electrons and there's 1 pi electron per carbon (so 4 in total).
Filling up from the bottom, since fewer nodes means lower energy (generally), you have the n=1 and n=2 states filled and the others unfilled. This means that the overall picture looks a bit like there's a big partial pi-bond across the whole molecule and then a set of two partial pi-bonds between the outer pairs of atoms (i.e. 1 with 2 and 3 with 4).
...but that's the MO treatment of conjugated systems.
Related discussions
- Should I apply to Cambridge?
- EPQ ideas - Physics
- A level mechanics help
- Moments
- Astrophysics
- Mechanics A Level Maths Question
- Trigonometric Modelling
- Moles in A-Level Chemistry
- As mechanics question
- Physics personal statement advice
- AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 25th May 2023 [Exam Chat]
- as level mechanics suvat
- Need help with kinematic question (AS)
- Uni physics advice
- ladders!!! - M2
- GCSE AQA Physics Paper 1 and 2 Revision and Study Chat
- Can someone help me understand these definitions in mechanics?
- Physics or Computer science?
- m1 june 2002 paper
- Gcse physics mocks
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



