AQA CHEM4 - 13th June 2012
Scroll to see replies
Topics I'm really scared for is as follows:
rate equations
equilibrium constant
acids, bases and pH calculations
pH curves, titration and indicators
titration calculation
BUFFER BSSSSS

can someone post every related question to this topic onto here from 2002 and we'll completely dominate these types of questions.
rate equations
equilibrium constant
acids, bases and pH calculations
pH curves, titration and indicators
titration calculation
BUFFER BSSSSS

can someone post every related question to this topic onto here from 2002 and we'll completely dominate these types of questions.
Original post by Casshern1456
Topics I'm really scared for is as follows:
rate equations
equilibrium constant
acids, bases and pH calculations
pH curves, titration and indicators
titration calculation
BUFFER BSSSSS

can someone post every related question to this topic onto here from 2002 and we'll completely dominate these types of questions.
rate equations
equilibrium constant
acids, bases and pH calculations
pH curves, titration and indicators
titration calculation
BUFFER BSSSSS

can someone post every related question to this topic onto here from 2002 and we'll completely dominate these types of questions.
How are you finding NMR?
What are you finding hard in the topics you said? I'll try help

Original post by Doctor.
I agree with the "-N-Trimethyl" its the bit before that, I disagree with though.
Where are you getting the Propanoic acid from? That part could have been been an Aldehyde?
I would say its Propanamide N-trimthyl
Nice that covers the CH3CH2CON bit and ofcoure the N-(CH3)3 bit is correct?
That's just what I think though
Where are you getting the Propanoic acid from? That part could have been been an Aldehyde?
I would say its Propanamide N-trimthyl
Nice that covers the CH3CH2CON bit and ofcoure the N-(CH3)3 bit is correct?
That's just what I think though

you're correct, my bad. I would write it N-methyl propanamide though. Propanamide because its essentially an aldehyde with a amine group attached. got it.
Original post by callmenighthawk
you're correct, my bad. I would write it N-methyl propanamide though. Propanamide because its essentially an aldehyde with a amine group attached. got it.
Yeah I see what you mean, I hate naming it when they hace the N- makes everything complicated

Original post by Doctor.
Yeah I see what you mean, I hate naming it when they hace the N- makes everything complicated 

I think of the N as like a number - like 1-methyl etc, I'm not sure if it works.
Original post by MuffinMonster
I think of the N as like a number - like 1-methyl etc, I'm not sure if it works.
Kinda, except the N just basically says what is on the N, rather than saying where it is (because obviously there is only one N in the molecule)
Original post by shuaib786
Can someone explain on how to do the proton nmr stuff like working out integration values and integration ratios???
You'll be given the integration values in the exam so don't worry about that. To get the ratios, just get the lowest common multiple between the numbers and you're done.
Original post by Doctor.
You'll be given the integration values in the exam so don't worry about that. To get the ratios, just get the lowest common multiple between the numbers and you're done.
So thanks how would you do the 8 c) i) and ii) from the chem 4 jan 2006 paper from the old spec.
Original post by shuaib786
So thanks how would you do the 8 c) i) and ii) from the chem 4 jan 2006 paper from the old spec.
I'll have a look at it tomorrkw, but be a little wary the old spec did have emphasis on certain topics and less emphasis on others.
Original post by xiyangliu
Unit 5..... So much memorising compare to unit 4 !! Any better ways to revise unit 5 ???
This was posted from The Student Room's iPhone/iPad App
This was posted from The Student Room's iPhone/iPad App
Unit 5 isn't much memorising tbh. It's all about understanding what it going on. So make sure you get exactly what is happening, then question yourself..."but why is it doing that?" - it helps!!!
Original post by Doctor.
I'll have a look at it tomorrkw, but be a little wary the old spec did have emphasis on certain topics and less emphasis on others.
yh plz

Original post by shuaib786
yh plz 

Sorry didnt manage to take a look today. Had a ton of other revision to do. Will try take a look tomorrow
 again sorry!
again sorry!ANYONE HAVE THE JAN 2012 UNIT 4 CHEM PAPER ??!!
pleaseeeeeeeee
pleaseeeeeeeee
Original post by rss.914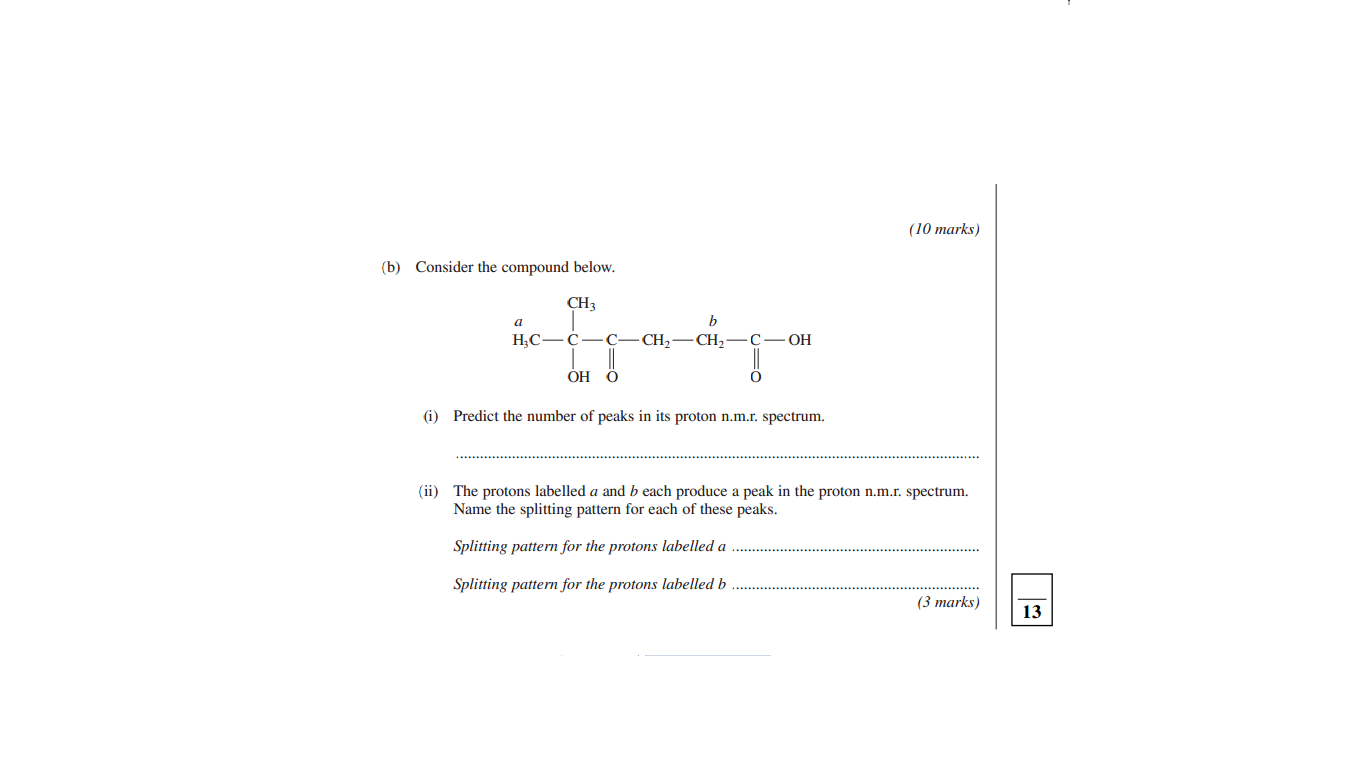
anyone know the answer to the number of peaks
anyone know the answer to the number of peaks
five I think
Original post by Mero8
five I think
how did you get 5? thats the right answer. ii could only get 4
Original post by rss.914
how did you get 5? thats the right answer. ii could only get 4
because you get 5 groups starting from the most electronegative substance the oxygen, so when you count the protons at every different occurring position along the chain the Hydrogen it is in will affect how many peaks there are. The 2 CH3 group on the second Carbon count as 1 for example.
(edited 11 years ago)
Original post by Casshern1456
because you get 5 groups starting from the most electronegative substance the oxygen, so when you count the protons at every different occurring position along the chain the Hydrogen it is in will affect how many peaks there are. The 2 CH3 group on the second Carbon count as 1 for example.
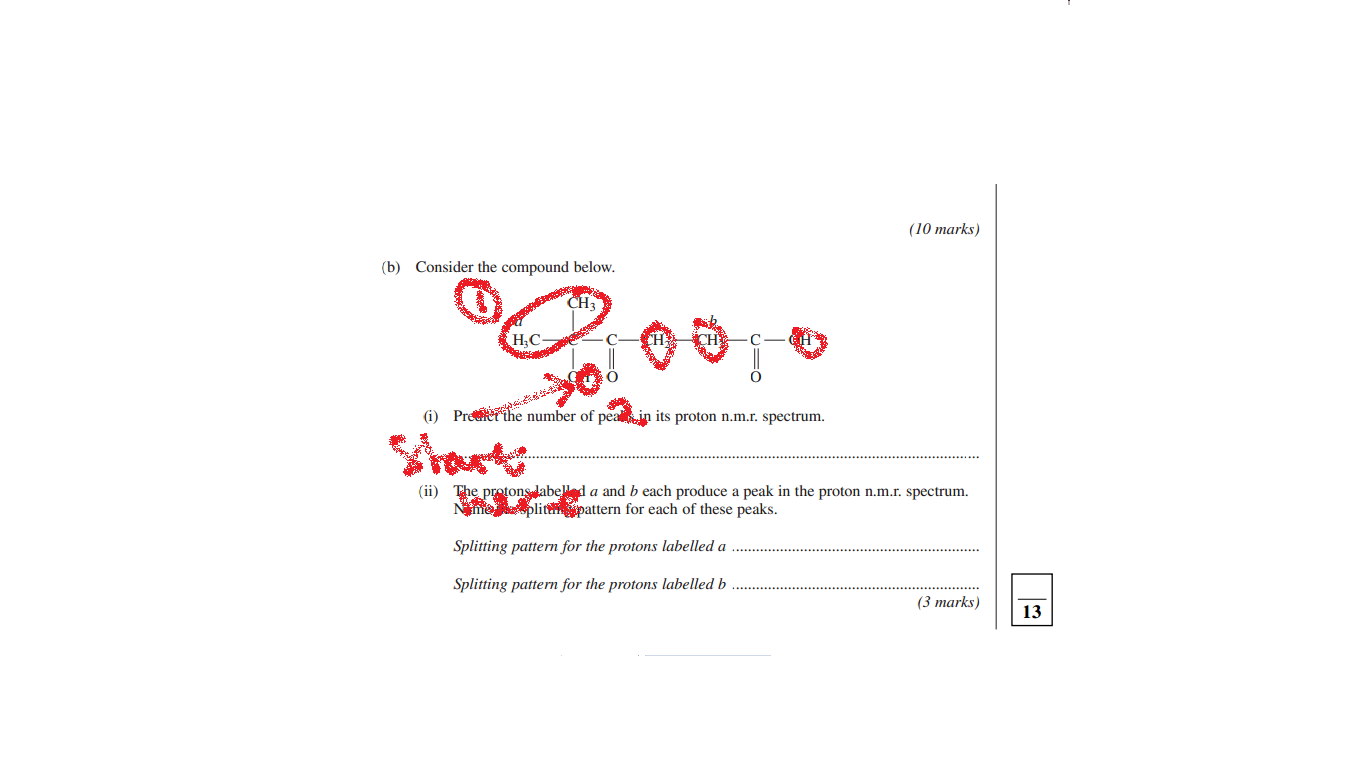
thanks but why don't the two ch2 groups count as one?
Quick Reply
Related discussions
- GCSE Exam Discussions 2024
- GCSE Exam Discussions 2023
- NEED HELP with KC equilibrium question please!
- AQA GCSE Combined Science Paper 2 Foundation (8464/C/2F) - 13th June 2023 [Exam Chat]
- AQA GCSE Physical sciences (Higher Combined) 8465/4H - 13th June 2023 [Exam Chat]
- AQA GCSE Chemistry Paper 2 Higher Tier (8462/2H) - 13th June 2023 [Exam Chat]
- AQA GCSE Spanish Writing Foundation Tier 4F (8698/WF) - 13th June 2023 [Exam Chat]
- A Level Exam Discussions 2023
- A-level Exam Discussions 2024
- GCSE Biology Study Group 2022-2023
- AQA GCSE Chemistry Paper 2 Foundation Tier (8462 2F) - 13 June 2023 [Exam Chat]
- AQA GCSE Spanish Writing Higher Tier 4H (8698/WH) - 13th June 2023 [Exam Chat]
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- Go ahead in time as far as possible
- A-level History Study Group 2022-2023
- Using ATAS certificate twice
- GCSE November Series 2023 Exam Discussions Hub
- Edexcel GCSE Combined Sci Paper 2 Foundation (1SC0 2CF) - 13th June 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- AQA GCSE Mathematics Paper 3 (8300/3) - 13th November 2023 [Exam Chat]
Latest
Trending
Last reply 18 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 18 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



