Confused about 'Chemical Bonding'
Hi,
I'm in year 11 and have the rest of my Gcse's to complete this summer. I've revised a lot for biology today, and now I've come to do chemistry and I keep finding myself getting confused when I'm reading about chemical bonding. I can't remember 'explaining physical properties' of ionic compounds, of covalent bonds, or of Giant covalent substances; in fact, I'm not even 100% sure what they even are. I want to be really confident for my exam, and make sure I know the required information like the back of my hand. I'm just having a few troubles remembering these things...any advice please anyone?
Posted from TSR Mobile
I'm in year 11 and have the rest of my Gcse's to complete this summer. I've revised a lot for biology today, and now I've come to do chemistry and I keep finding myself getting confused when I'm reading about chemical bonding. I can't remember 'explaining physical properties' of ionic compounds, of covalent bonds, or of Giant covalent substances; in fact, I'm not even 100% sure what they even are. I want to be really confident for my exam, and make sure I know the required information like the back of my hand. I'm just having a few troubles remembering these things...any advice please anyone?

Posted from TSR Mobile
Original post by 3mma_gal96
xx
Chemistry is all about adding A to B to make C. Chemical bonding is how that is achieved. A bond can be considered as A and B sharing (or giving) an electron.
Covalent bonding is where A and B share an electron to form a bond (look at the dot and cross diagram below)
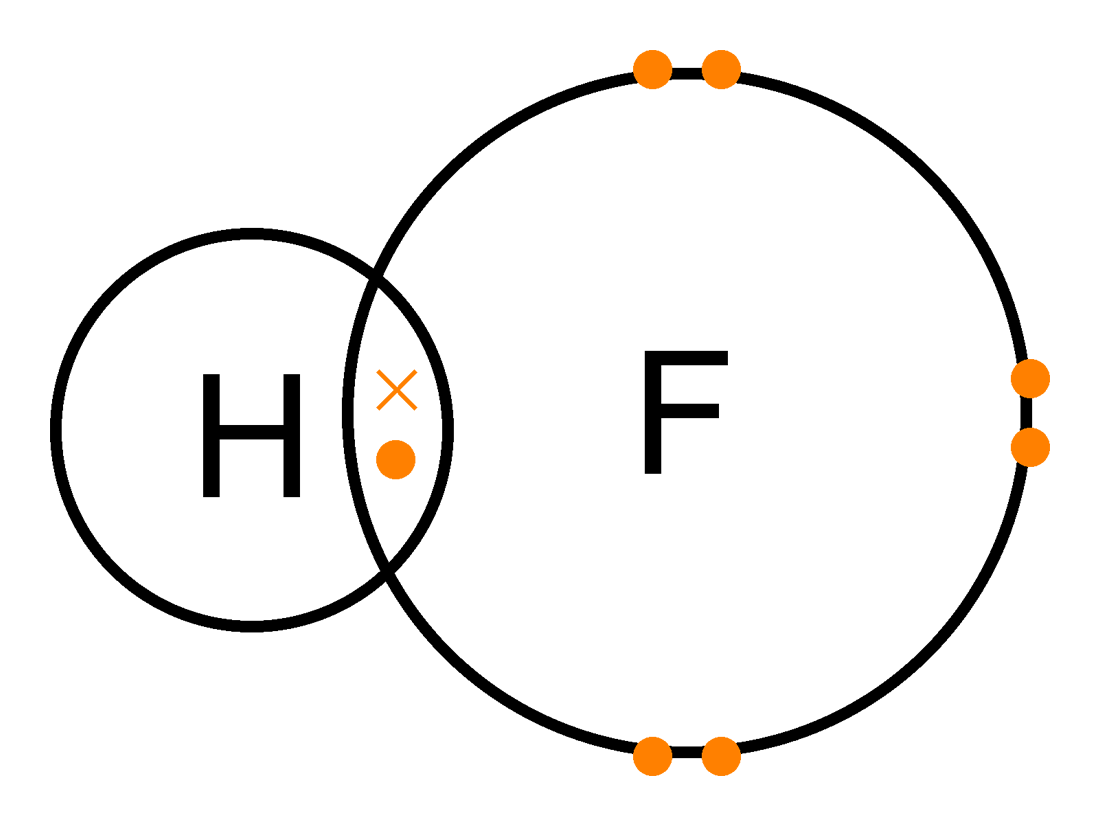
Ionic Bonding is where A gives an electron to B. This will make A positively charged and B negatively charged, and then A and B attract each other, almost like a magnet.

Giant Covalent structures are exactly what you might expect from the name. They are covalent structures (so they share their electrons to form bonds) but there are lots of them so they form GIANT structures! The classic example of this is diamond (which goes on forever).

Original post by Nirgilis
Chemistry is all about adding A to B to make C. Chemical bonding is how that is achieved. A bond can be considered as A and B sharing (or giving) an electron.
Covalent bonding is where A and B share an electron to form a bond (look at the dot and cross diagram below)

Ionic Bonding is where A gives an electron to B. This will make A positively charged and B negatively charged, and then A and B attract each other, almost like a magnet.

Giant Covalent structures are exactly what you might expect from the name. They are covalent structures (so they share their electrons to form bonds) but there are lots of them so they form GIANT structures! The classic example of this is diamond (which goes on forever).

Covalent bonding is where A and B share an electron to form a bond (look at the dot and cross diagram below)

Ionic Bonding is where A gives an electron to B. This will make A positively charged and B negatively charged, and then A and B attract each other, almost like a magnet.

Giant Covalent structures are exactly what you might expect from the name. They are covalent structures (so they share their electrons to form bonds) but there are lots of them so they form GIANT structures! The classic example of this is diamond (which goes on forever).

Ahh ok, thanks, makes a lot more sense now

Posted from TSR Mobile
Original post by 3mma_gal96
To further quote the helper's post, here are some common misconceptions students tend to have.
1) Ionic bond - electrostatic force of attraction between oppositely charged ions
ionic bonding - the process involving electron transfer that results in formation of ions that interact to form the ionic bond
2) despite drawing dot and cross diagram of sodium chloride, NaCl as some sort of discrete compound (i.e. one NaCl on its own), truth is that NaCl is never found that way. It exists as a lattice of ions held together by ionic bonds. Take note though, in those lattice diagrams, those lines joining adjacent ions do not represent ionic bonds, as again, ionic bonds are simply force of attraction!
Name a type of bonding not present in elements.
Explain why ionic compounds are soluable in water.
Explain why ionic compounds conduct electricity in molten and aqueous state but not in solid state.
Describe or draw the structure of NaCl lattice.
Explain why ionic compounds are brittle.
Explain why ionic compounds possess high melting temperatures (points)
Explain why ionic aqueous solutions undergo electrolysis.
Suggest ways in which ionic compounds differ from metallic and coavalent compounds. Explain your answers.
Draw dot and cross symbols for simple coavalent compounds.
Explain why simple covalent substances possess low melting points, are insoluble in water, do not conduct electricity, etc.
Draw the structures of diamond and graphite.
explain why diamond and graphite have high sublimation points (melting points).
Explain why only graphite conducts electricity and not diamond.
Explain why diamond is hard.
By referring to the structure and properties of diamond and graphite explain some of their uses.
Describe how metals form metallic bonding.
Explain why metals conduct electricity and heat, are malleable and ductile using their structures.
What is a covalent bond
That's pretty much what you can get !
Remember when explaining why substances conduct electricity refer to free mobile electrons which can move through the structure not just free electrons
Explain why ionic compounds conduct electricity in molten and aqueous state but not in solid state.
Describe or draw the structure of NaCl lattice.
Explain why ionic compounds are brittle.
Explain why ionic compounds possess high melting temperatures (points)
Explain why ionic aqueous solutions undergo electrolysis.
Suggest ways in which ionic compounds differ from metallic and coavalent compounds. Explain your answers.
Draw dot and cross symbols for simple coavalent compounds.
Explain why simple covalent substances possess low melting points, are insoluble in water, do not conduct electricity, etc.
Draw the structures of diamond and graphite.
explain why diamond and graphite have high sublimation points (melting points).
Explain why only graphite conducts electricity and not diamond.
Explain why diamond is hard.
By referring to the structure and properties of diamond and graphite explain some of their uses.
Describe how metals form metallic bonding.
Explain why metals conduct electricity and heat, are malleable and ductile using their structures.
What is a covalent bond
That's pretty much what you can get !
Remember when explaining why substances conduct electricity refer to free mobile electrons which can move through the structure not just free electrons
Quick Reply
Related discussions
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio)
- bio help a level
- Electrostatic forces
- Please help me find marks (2 marks of an A* for A level chemistry)
- what do the empty sticks mean in the chemistry data sheet?
- Help urgent spectroscopy
- What type of isomerism do Ketones and Functional groups have?
- Alevel Chemistry Aromatic Compounds
- chemical bonding
- enthalpy changes
- SN1 and SN2 reactions (chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- Polarising effect and lattice enthalpy
- Check my AS bio answer (mark scheme)
- Chem alevel help
- bond enthalpy question
- Help chemistry urgent
- Learndirect- presentation advice
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Weak Bases - Names, Properties and Examples
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



