Official AQA AS Chemistry Unit 1 - 23Rd May 2013
Hi Everyone,
Thought I'd create a thread for everyone to discuss about the chemistry exam this week.
Any predictions, revision tips and help can be posted here!
Good luck everyone!!
Thought I'd create a thread for everyone to discuss about the chemistry exam this week.
Any predictions, revision tips and help can be posted here!
Good luck everyone!!
Scroll to see replies
Wooooooooooooooooooooo

Original post by The.Joker
I'm gonna have my ion this thread... 

Smart.

Original post by The.Joker
I'm gonna have my ion this thread... 

I lol'ed.
Original post by Manni
Anyone got any good notes on bonding? Any tips on how to easily identify which kind of forces are acting upon certain elements
It's just non-metal-non-metal bond = Covalent
Metal-non-metal = Ionic
Metal-metal = Metallic

Hydrogen bonds between H + (N,F,O) - Strongest IMF.
Permanent dipole - I think it's C-Cl bonds and stuff...
VDW - Weakest IMF.
Hmm.
Original post by buzzing22
Anybody got any ideas on key topics to come up?
This
+
Since i'm new i thought I'll contribute;
http://www.youtube.com/playlist?list=PLXHv_GPXxvVD18kqY3O3yF3mJzSsts6Rl
(edited 10 years ago)
Original post by The.Joker
I'm gonna have my ion this thread... 

Hahaha!!!
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?

Original post by buzzing22
Anybody got any ideas on key topics to come up?
This + Since I'm new thought I'll contribute;
http://www.youtube.com/playlist?list=PLXHv_GPXxvVD18kqY3O3yF3mJzSsts6Rl
Original post by tumblrgirl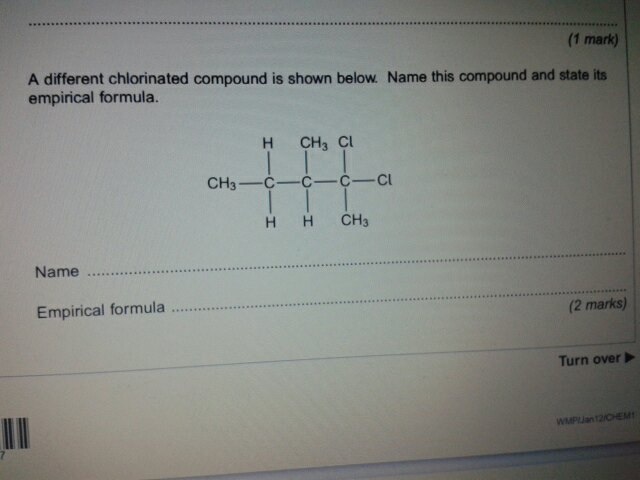
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?

Longest carbon chain is 5 so PENT
No C=C so ANE
2Chlorines on Carbon2 So 2,2 Dichloro
1 Methyl group on Carbon 3 so 3 Methyl
Add them together in alphabetical order 2,2-Dichloro-3-MethylPentane
Original post by tumblrgirl
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?
so I came across this question in Jan12 paper and I got a completely different answer from the markscheme on what the name of it is...
the markscheme says it's called 2,2 dichloro 3 methyl pentane....i don't understand why, anyone wanna help me?

It's pent- because the longest chain is five carbons (it's not straight it's goes 'round the corner' the CH3 group on the bottom right is carbon number 1 if you get me). The chlorines are both on the second carbon and then there is a methyl group on the third carbon.
That's actually quite complicated when you look at it, I definitely wouldn't have got that answer :L
ooh thanks guys, i'm so silly, i didn't realise that there were 5 carbons -_-
thanks again
thanks again

If anybody has other exam questions they are stuck on, feel free to post, Would benefit yourself and everyone else
this sounds really stupid but how do you know which carbon you would say is carbon 1, etc?
watchin, done no revision so far, im doing c2 at the moment
Original post by tumblrgirl
this sounds really stupid but how do you know which carbon you would say is carbon 1, etc?
Take this for example
You would deduce which way you look at it gives you the smallest number on the carbon chain, from left to right it would be 4,4-DiChloro-3-MethylPentane. You then would look at it from the other way (right to left) and it would be 2,2-DiChloro-3-MethylPentane.
As you can see the position of the functional groups on the 2nd Haloalkane is lower than the 1st Haloalkane so that would be it.
Hope this makes sense.
(edited 10 years ago)
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- A-level Business Study Group 2022-2023
- Edexcel chemistry unit 2 mixed questions
- AQA A Level Law Paper 1 (7162/1) - 23rd May 2024 [Exam Chat]
- WJEC A Level History Paper 1 - 23rd May 2024 [Exam Chat]
- OCR AS-Level History Unit 2 (Y243,Y249,Y251-253) - 23rd May 2023 [Exam Chat]
- A-level Chemistry Study Group 2022-2023
- School is killing me - Y11 "GYG" 2022
- SQA Exam Discussions 2024
- AQA A Level History Paper 1 (7042/1A-1L) - 23rd May 2024 [Exam Chat]
- Self-teaching Chemistry A-level (as a private candidate)?
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- WJEC A Level Business A2 Unit 3 (1510U30-1) - 23rd May 2023 [Exam Chat]
- UNIQ 2024 Participants Thread
- A-level History Study Group 2022-2023
- AQA A-level English Language Paper 1 (7702/1) - 23rd May 2024 [Exam Chat]
Latest
Trending
Last reply 12 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 12 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



