Some AS/A2 Chemistry questions
Recently, I've been trying to review some of AS Chemistry for a test on a section of A2 Chemistry but things just aren't clicking in my head so here are some questions I'd like answered..
1) How are we supposed to know if there is dative covalent bonding in a molecule? Why is there dative covalent bonding in CO and can someone please explain the 'mechanism' behind dative covalent bonding in an ammonium ion?
2) In the oxidation of alcohols, where is the OILRIG demonstrated, e.g. when primary alcohols are oxidised to aldehydes and then to carboxylic acids?
3) Why is ammonia a base?
1) How are we supposed to know if there is dative covalent bonding in a molecule? Why is there dative covalent bonding in CO and can someone please explain the 'mechanism' behind dative covalent bonding in an ammonium ion?
2) In the oxidation of alcohols, where is the OILRIG demonstrated, e.g. when primary alcohols are oxidised to aldehydes and then to carboxylic acids?
3) Why is ammonia a base?
2).
 you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal
you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal3) Ammonia is considered a base because it is able to accept a H+ ion , so NH3 will go onto from NH4+, so the NH3 donates a lone pair of electrons to form a bond with H+, base is basically an electron donator
(edited 10 years ago)
Original post by mattcandy3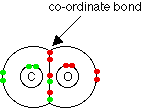 CO is considered a dative covalent bond, because normally in covalent bonds, you have 1 electron from each atom contributing to the covalent bond, however in this particular case, you have 2 electrons being donated from the oxygen, all a dative covalent bond is when 2 electrons are donated from the same atom. In a dative covalent bond , one atom provides both the electrons
CO is considered a dative covalent bond, because normally in covalent bonds, you have 1 electron from each atom contributing to the covalent bond, however in this particular case, you have 2 electrons being donated from the oxygen, all a dative covalent bond is when 2 electrons are donated from the same atom. In a dative covalent bond , one atom provides both the electrons
2). you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal
you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal
3) Ammonia is considered a base because it is able to accept a H+ ion , so NH3 will go onto from NH4+, so the NH3 donates a lone pair of electrons to form a bond with H+, base is basically an electron donator
2).
 you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal
you would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromate(VI) solution acidified with dilute sulphuric acid., in these terms, it is oxidising as you have lost a hydrogen to change ethanol, to ethanal3) Ammonia is considered a base because it is able to accept a H+ ion , so NH3 will go onto from NH4+, so the NH3 donates a lone pair of electrons to form a bond with H+, base is basically an electron donator
Thanks but for questions 1 and 2, you haven't really answered what I was looking for.
Why is there dative covalent bonding in CO rather than just covalent bonding? Is it possible to tell whether a molecule is dative covalently bonded by just being given the formula? For number 2, why is removing the hydrogen oxidising? Is it because a hydrogen contains one electron and therefore that is the equivalent of losing a electron in the OIL of OILRIG?
1) there has to be dative covalent bond, because, if you look at the molecule , each atom has to gain a full outer shell of electrons e.g 8 electrons, carbon has 4 outer shell electrons and oxygen 6, therefore, if carbon shares 2 of its electrons and oxygen also share 2 of its electrons, then this leaves the oxygen with 8 outer shell electrons (see picture above, red dots are oxygen electrons and green are carbon electrons), but unfortunately carbon with have only 6, so therefore to get that to 8, oxygen, has to share 2 of it own electrons with carbon, hence giving the 8-8 outer shell for both carbon and oxygen, however the 2 oxygen electrons haven't bonded with carbon electrons, because there is no carbon electrons left to share as they have already covalently bonded with some of the oxygen electrons , therefore both electrons have to come from the oxygen, hence a dative covalent, bond which the definition, is shared pair of electrons that both come from the same atom. Some molecules such as NH4 you should learn that they are dative covalent bonded, however no to test if they are you will have to draw out the dot and cross diagram.
2) Removing of the hydrogen is oxidation, because it is removal of electrons, oil rig, oxidation is lost, and reduction is gain, and in this the atom is losing an electron from the hydrogen, to leave you with ethanal. If you need further explaination just ask.
2) Removing of the hydrogen is oxidation, because it is removal of electrons, oil rig, oxidation is lost, and reduction is gain, and in this the atom is losing an electron from the hydrogen, to leave you with ethanal. If you need further explaination just ask.
(edited 10 years ago)
Original post by mattcandy3
1) there has to be dative covalent bond, because, if you look at the molecule , each atom has to gain a full outer shell of electrons e.g 8 electrons, carbon has 4 outer shell electrons and oxygen 6, therefore, if carbon shares 2 of its electrons and oxygen also share 2 of its electrons, then this leaves the oxygen with 8 outer shell electrons (see picture above, red dots are oxygen electrons and green are carbon electrons), but unfortunately carbon with have only 6, so therefore to get that to 8, oxygen, has to share 2 of it own electrons with carbon, hence giving the 8-8 outer shell for both carbon and oxygen, however the 2 oxygen electrons haven't bonded with carbon electrons, because there is no carbon electrons left to share as they have already covalently bonded with some of the oxygen electrons , therefore both electrons have to come from the oxygen, hence a dative covalent, bond which the definition, is shared pair of electrons that both come from the same atom. Some molecules such as NH4 you should learn that they are dative covalent bonded, however no to test if they are you will have to draw out the dot and cross diagram.
2) Removing of the hydrogen is oxidation, because it is removal of electrons, oil rig, oxidation is lost, and reduction is gain, and in this the atom is losing an electron from the hydrogen, to leave you with ethanal. If you need further explaination just ask.
2) Removing of the hydrogen is oxidation, because it is removal of electrons, oil rig, oxidation is lost, and reduction is gain, and in this the atom is losing an electron from the hydrogen, to leave you with ethanal. If you need further explaination just ask.
Thank you!
I hope you don't mind answering another question: could you please explain why it is not hydrogen cyanide joining onto the alcohol in the first stage in the addition mechanism but cyanide ions? Is it because of the H+ and CN-?
i don't know what your referring to, its to vague what you asked can you please explain?
Original post by mattcandy3
i don't know what your referring to, its to vague what you asked can you please explain?
In the addition mechanism of, say, aldehydes and hydrogen cyanide, when you draw the diagram with the curly arrows, it only shows the CN joining onto the aldehyde (with the carbon of the CN attacking the carbon in the carbonyl group and the double bond breaking etc.). Where did the hydrogen from the hydrogen cyanide go or did it dissolve in water to dissociate into H+ and CN- ions?
Also, in the final stage of the mechanism, a proton (H+) joins onto the negatively charged oxygen - does this come from the hydrogen of the hydrogen cyanide or the H+ ions in the water or both?
Another question: why is ethanol a weaker acid than water? In fact, why is it a weak acid after all?
It is a weaker acid , because it loses a proton from the hydroxyl group,, its weaker then water because the ethoxide ion is a stronger base than the hydroxide ion therefore overall it will be weaker, apart from methanol. Methanol is the exception it is slightly more acidic then water, Water is also a better proton donor than alcohol,
Once again, i really dont understand what your trying to explain, is there a question you can show me etc,?
Once again, i really dont understand what your trying to explain, is there a question you can show me etc,?
Quick Reply
Related discussions
- I want to study astrophysics but need help picking A-levels
- How do I prepare for an Oxford Chemistry interview?
- Life and Health science (step up science equivalent)
- HELP im horrible at module 2 OCR chemistry
- What A Levels are required to apply for bioinformatics?
- Study resources for edexcel IAL
- What if I'm doing 4 A Levels but the actual offer from universities only require 3?
- Uclan university pharmacy
- Self-teaching Chemistry A-level (as a private candidate)?
- Applying to medicine, bad IGCSE results.
- a level biology and chemustry
- The A-level grind
- Welcome to the A level forum!
- Inorganic Chemistry (AQA) - A level
- alevel biology and chemistry
- Biology degree without chemistry A-level?!
- 5 A Levels too much?
- Can I learn Alevel bio and chem AS/A2 content in 5 months in prep for 2024 exams?
- Resitting AS - advice needed
- AS Chemistry Paper 1 2022
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



