hydration of ethene to make ethanol
just wondering if this applies to all alkenes i.e. if we take any alkene and react it with water with a conc. phosphoric acid catalyst will it give us the alcohol?
Original post by TheGreaterGood
just wondering if this applies to all alkenes i.e. if we take any alkene and react it with water with a conc. phosphoric acid catalyst will it give us the alcohol?
If you start from an unsymmetrical alkene like propene, you have to be careful to think about which way around the water adds across the carbon-carbon double bond.
Markovnikov's Rule says that when you add a molecule HX across a carbon-carbon double bond, the hydrogen joins to the carbon atom which already has the more hydrogen atoms attached to it.
Thinking of water as H-OH, the hydrogen will add to the carbon with the more hydrogens already attached. That means that in the propene case, you will get propan-2-ol rather than propan-1-ol.

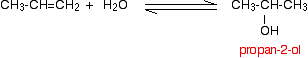
The conditions used during manufacture vary from alcohol to alcohol.
Original post by ApplyYourself
If you start from an unsymmetrical alkene like propene, you have to be careful to think about which way around the water adds across the carbon-carbon double bond.
Markovnikov's Rule says that when you add a molecule HX across a carbon-carbon double bond, the hydrogen joins to the carbon atom which already has the more hydrogen atoms attached to it.
Thinking of water as H-OH, the hydrogen will add to the carbon with the more hydrogens already attached. That means that in the propene case, you will get propan-2-ol rather than propan-1-ol.

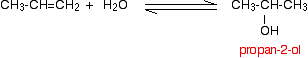
The conditions used during manufacture vary from alcohol to alcohol.
Markovnikov's Rule says that when you add a molecule HX across a carbon-carbon double bond, the hydrogen joins to the carbon atom which already has the more hydrogen atoms attached to it.
Thinking of water as H-OH, the hydrogen will add to the carbon with the more hydrogens already attached. That means that in the propene case, you will get propan-2-ol rather than propan-1-ol.

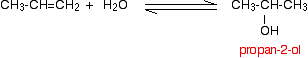
The conditions used during manufacture vary from alcohol to alcohol.
oh yeah ahh forgot about that rule
thank you

Original post by TheGreaterGood
oh yeah ahh forgot about that rule
thank you
thank you

NP

Quick Reply
Related discussions
- Chemistry question
- chemistry help
- chemistry moles question
- enthalpy question
- biotechnology
- enthalpy change of solution
- Mass Spectrometry question
- Hydrated Salts question
- AS Organic Chem Practical Question
- The heat released when 1.00 g of ethanol (Mr = 46.0) undergoes complete combustion is
- Daphnia heart rate Practical
- Is the test for lipids in food the Sudan III test or ethanol test?
- pls help me out, do it in details with formula
- Amount of substances
- a level chemistry
- chemistry a level ocr question
- GCSE Chemistry question
- chemistry
- Chemistry - alcohols
- Rate my skincare routine
Latest
Last reply 1 minute ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 minute ago
Plymouth Marjon University - Your Questions AnsweredLast reply 2 minutes ago
CPS Pupillage/Legal Trainee Scheme 2024 (2025 Start)Last reply 2 minutes ago
Government Social Research - Research Officer Scheme 2024Last reply 3 minutes ago
TSR Study Together - STEM vs Humanities - Tenth SessionLast reply 4 minutes ago
Official University College London Applicant Thread for 2024Last reply 5 minutes ago
HSBC Degree Apprenticeship 2024Last reply 6 minutes ago
which unis will accept BBB for economicsLast reply 7 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 9 minutes ago
Edexcel GCSE Statistics Paper 2 Higher Tier (1ST0 2H) - 19th June 2023 [Exam Chat]Maths Exams
169
Last reply 10 minutes ago
Medical doctor degree apprenticeship 2024Trending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products



