Edexcel AS level Unit 3B: Chemistry Laboratory Skills I Alternative May 7 2014
Scroll to see replies


never came across that anti bumping granules thing, where did you see it?
its not there in a paper..its always used during distillation and reflux and i wondered why so i decided to ask y'áll
its always calcium as the "red" unless otherwise.. i remember in jan/june 10 they said clearly that a red flame is observed for a group 1 cation so in that case it was lithium
which one of the three halogenoalkane form ppt faster? primary, secondary or tertiary?
u forgot calcium too
om gosh i just called up my teacher to ask him this question.. its tertiary alcohol because the methyl groups weaken the C-X bond..the more the methyl groups the faster the reaction
hahaha XD thanx alot

CAN YOU PLEASE CHECK OUT JUNE 2010 Q2 A(iii) THE MARK SCEHME GAVE THE ANSWER AS A SECONDARY H.A IF IM NOT MISTAKEN.
yeah because in that case the only 2 HA are primary and secondary.. lol because C3H7I cannot form tertiary halogenoalkane hence the only options are primary and secondary ...so secondary...
if u had a halogenoalkane with -but then its possible so you would say tertiary...
If u had a halogenoalkane with -but then its possible so you would say tertiary...
ohhh okay. Excuse me for my stupidness
 thank you again
thank you againObviously, the time taken for a precipitate of silver halide to appear will depend on how much of everything you use and the temperature at which the reaction is carried out. But the pattern of results is always the same.
For example:
•
A primary iodo compound produces a precipitate quite quickly.
•
A primary bromo compound takes longer to give a precipitate.
•
A primary chloro compound probably won't give any precipitate until well after you have lost interest in the whole thing!
The order of reactivity reflects the strengths of the carbon-halogen bonds. The carbon-iodine bond is the weakest and the carbon-chlorine the strongest of the three bonds. In order for a halide ion to be produced, the carbon-halogen bond has to be broken. The weaker the bond, the easier that is.
im not sure bout the primary, secondary and tertiary though :/
Comparing the reaction rates of primary, secondary and tertiary halogenoalkanes
You would need to keep the halogen atom constant. It is common to use bromides because they have moderate reaction rates. You could, for example, compare the reactivity of these compounds:
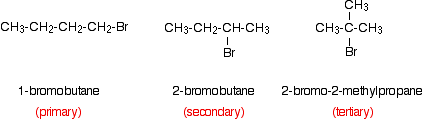
For example:
•
The tertiary halide produces a precipitate almost instantly.
•
The secondary halide gives a slight precipitate after a few seconds. The precipitate thickens up with time.
•
The primary halide may take considerably longer to produce a precipitate.
It is more difficult to explain the reason for this, because it needs a fairly intimate knowledge of the mechanisms involved in the reactions. It reflects the change in the way that the halide ion is produced as you go from primary to secondary to tertiary halogenoalkanes.
Suggest why removing excess solid drying agent by decanting, rather than filtering through filter paper, improves the yield.
suggest why removing excess solid drying agent by decanting, rather than filtering through filter paper, improves the yield.
because filter paper absorbs some of the product. And btw do we need to focus on mass spectra for unit 3?
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Chemistry degree
- Pharmacy/a levels
- Self-teaching Chemistry A-level (as a private candidate)?
- Component query
- iria's 'grow your grades' blog :)
- Access course for Pharmacy
- Gap Year A-Level Chemistry to go into Med.
- How many GCSEs are you getting?
- GCSE Results: Post your results
- Access to HE Science for Medicine
- GCSE French vs A-level French
- Edexcel AS level unit-1 and unit-2 business notes
- AS/A Level Chemistry Study Group 2023/2024
- Which exam boards are easiest to take Chemistry and Biology A-levels with?
- ..
- Resisting btec units
- Can 1st-year BTEC Applied Science qualify for Pharmacy at uni?
Latest
Last reply 2 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 3 minutes ago
Official University of Sheffield Offer Holders Thread for 2024 entryLast reply 3 minutes ago
HSBC Degree Apprenticeship 2024Last reply 3 minutes ago
emmanuella's study discussions #2: taking breaksLast reply 3 minutes ago
Official University College London Applicant Thread for 2024Last reply 4 minutes ago
Predicted Paper 1 English Literature 2024 GCSELast reply 5 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry Thread2858
Last reply 7 minutes ago
Michaela School: Muslim student loses prayer ban challengeLast reply 7 minutes ago
Official: Aston University A100 2024 Entry Applicant thread1130
Last reply 7 minutes ago
Edexcel A-level Spanish Paper 3, IRP/Speaking (9SP0 03) - 2024 [Exam Chat]Last reply 8 minutes ago
NICS Staff Officer and Deputy Principal recruitment 2022 2023Last reply 8 minutes ago
AQA A Level Sociology Paper 2 (7192/2) - 4th June 2024 [Exam Chat]Posted 10 minutes ago
Which uni to study at - LSE or KingsLast reply 13 minutes ago
BAE systems degree apprenticeships September 2024Last reply 14 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 15 minutes ago
yet to receive a woodhouse interview


