Edexcel AS level Unit 3B: Chemistry Laboratory Skills I Alternative May 7 2014
Scroll to see replies
Which ion in the flame test gives yellow-red?
Posted from TSR Mobile
Posted from TSR Mobile
Original post by Miffyy
whenever they mention yellow red in brakets they also write (Brick Red) which automatically tells you its Calcium ion

no one has notes? or at least can someone list the experiments? 

guys new question from may 13
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
Original post by Paras Agarwal
guys new question from may 13
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
also why draw a cooling curve...i know what it is i need the explanation..helppp
Original post by Paras Agarwal
guys new question from may 13
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
1. take several measurements of temp of cuso4 solution before adding Zn metal
2. at 3.5 mins exactly add zn and continue taking the measurements of temp for 10 mins.
3. draw a graph of temp. and time
4.extrapolate graph at exactly 3.5 mins to get the highest temp that could be reached.
soo i guess , if i understood correctly, that instead of having a stop watch in ur hand , and putting Zn powder into the solution and then quickly hold the thermometer and measure initial and final temp (which will be inaccurate ) , just use a graph to extrapolate which will be much more accurate. As the first method u wont be fast enough and accurate enough to measure the highest temp reached (final temp.)
i hope u understood something
 i explain really bad
i explain really bad 
Original post by sorabackwards
no one has notes? or at least can someone list the experiments? 

Hey i have some notes ! Learn everything i've attached and don't worry.
Original post by mr osaka
1. take several measurements of temp of cuso4 solution before adding Zn metal
2. at 3.5 mins exactly add zn and continue taking the measurements of temp for 10 mins.
3. draw a graph of temp. and time
4.extrapolate graph at exactly 3.5 mins to get the highest temp that could be reached.
soo i guess , if i understood correctly, that instead of having a stop watch in ur hand , and putting Zn powder into the solution and then quickly hold the thermometer and measure initial and final temp (which will be inaccurate ) , just use a graph to extrapolate which will be much more accurate. As the first method u wont be fast enough and accurate enough to measure the highest temp reached (final temp.)
i hope u understood something i explain really bad
i explain really bad 
2. at 3.5 mins exactly add zn and continue taking the measurements of temp for 10 mins.
3. draw a graph of temp. and time
4.extrapolate graph at exactly 3.5 mins to get the highest temp that could be reached.
soo i guess , if i understood correctly, that instead of having a stop watch in ur hand , and putting Zn powder into the solution and then quickly hold the thermometer and measure initial and final temp (which will be inaccurate ) , just use a graph to extrapolate which will be much more accurate. As the first method u wont be fast enough and accurate enough to measure the highest temp reached (final temp.)
i hope u understood something
 i explain really bad
i explain really bad 
ohhh now i get it! thanks bruh!

Original post by Paras Agarwal
guys new question from may 13
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
Using the same equipment, together with a stop clock, suggest a procedure
that would improve the accuracy of this experiment by obtaining a more accurate
temperature change. You must use the same mass of zinc powder and the same
volume of 1.00 mol dm–3 copper(II) sulfate solution.
like can you please explain step by step properly on what to do and not just list points from the mark scheme.. this question is weeeird!
thanks
•pipette out 50cm3 of copper (ll) sulfate into a polystyrene cup and start stopwatch and measure the temperature simultaneously.
•record the temperature every 3 minutes. At 3.5 minute add solid and stir the mixture.
•then measure temperature from 4th minute to 10th minute.
•plot a graph of temperature against time. Connect the points before addition of solid and extrapolate it to the time of adding the solid. Connect the points after adding the solid and extra Polate back to the time of adding
Posted from TSR Mobile
Original post by angelom1994
Hey i have some notes ! Learn everything i've attached and don't worry.
ty so much :3 i already have 2 of em but the others i didnt so really a great help (y) thanks
Can someone please answer all these questions?

Posted from TSR Mobile
Original post by Miffyy
•pipette out 50cm3 of copper (ll) sulfate into a polystyrene cup and start stopwatch and measure the temperature simultaneously.
•record the temperature every 3 minutes. At 3.5 minute add solid and stir the mixture.
•then measure temperature from 4th minute to 10th minute.
•plot a graph of temperature against time. Connect the points before addition of solid and extrapolate it to the time of adding the solid. Connect the points after adding the solid and extra Polate back to the time of adding
Posted from TSR Mobile
•record the temperature every 3 minutes. At 3.5 minute add solid and stir the mixture.
•then measure temperature from 4th minute to 10th minute.
•plot a graph of temperature against time. Connect the points before addition of solid and extrapolate it to the time of adding the solid. Connect the points after adding the solid and extra Polate back to the time of adding
Posted from TSR Mobile
okay thank you!

new question from JAN 14! the IR SPECTRUM! does anyone have any logical proper wonderful explanation as to why the spectrum is for Hex-5-en-1ol and not for the cycloalkene??? i mean it could correspond to both but the cycloalkene is like 90 percent correct in my opinion
but the mark scheme says its the latter help?
help?
but the mark scheme says its the latter
 help?
help?Original post by Miffyy
((( Answers for first page )))
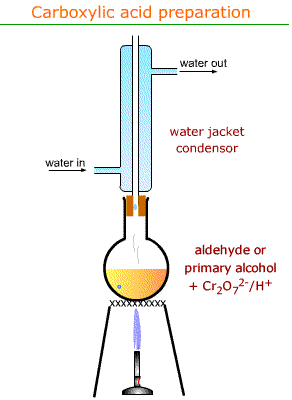
1- method is distillation so use the distillation drawing

equation is
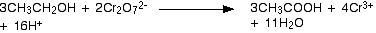
2-heat under reflux
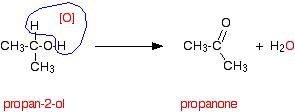
b) 1)
Original post by Paras Agarwal
new question from JAN 14! the IR SPECTRUM! does anyone have any logical proper wonderful explanation as to why the spectrum is for Hex-5-en-1ol and not for the cycloalkene??? i mean it could correspond to both but the cycloalkene is like 90 percent correct in my opinion
but the mark scheme says its the latter help?
help?
but the mark scheme says its the latter
 help?
help?I had the same question. Let's take it from the top. The largest peak corresponds to O-H so could be either, then the second largest = C-H , again could be either then there's a third one on the far right which corresponds to C=C . And i guess that eliminates the cyclic alcohol because it only has single C-C bonds
Learning how to spell phenolphthalein is my biggest personal achievement this year
Original post by Dangel
@Miffyy my answers are being checked by moderators, sorry !! 

Can you inbox it to me?

Posted from TSR Mobile
Original post by Paras Agarwal
guys!!!
do u know why we use anti bumping granules when distilling or doing reflux
do u know why we use anti bumping granules when distilling or doing reflux
it is inorder to ensure that the distrubtion of the heat is equally in the solution or the mixture being heated up
(edited 9 years ago)
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Chemistry degree
- Pharmacy/a levels
- Self-teaching Chemistry A-level (as a private candidate)?
- Component query
- iria's 'grow your grades' blog :)
- Access course for Pharmacy
- Edexcel A-level French Paper 3, IRP/Speaking (9FR0 03) - 2024 [Exam Chat]
- Gap Year A-Level Chemistry to go into Med.
- How many GCSEs are you getting?
- GCSE Results: Post your results
- Access to HE Science for Medicine
- GCSE French vs A-level French
- Edexcel AS level unit-1 and unit-2 business notes
- Which exam boards are easiest to take Chemistry and Biology A-levels with?
- GCSE Results Day 2022 - Retake and Remark Information
- AS/A Level Chemistry Study Group 2023/2024
- ..
Latest
Last reply 2 minutes ago
Woodhouse College applicants 2024Last reply 2 minutes ago
Name a girl's name which starts with the last letter of the above nameForum games
4615
Last reply 2 minutes ago
student finance and nhs fund for 2nd healthcare degree?Last reply 3 minutes ago
November 2023 Illegal Migration Intake Unit - Intake Response Team - Immigration OffiLast reply 3 minutes ago
Official MPhil Economics/Economic Research etc Cambridge 2024 ThreadLast reply 5 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 6 minutes ago
The Official Funding questions/moans/possible joy ThreadLast reply 7 minutes ago
BAE systems degree apprenticeships September 2024Last reply 9 minutes ago
LSE september 2024 grad intakeLast reply 9 minutes ago
Unsure of where to find student finance breakdownLast reply 12 minutes ago
Official University College London Applicant Thread for 2024Last reply 12 minutes ago
HSBC Degree Apprenticeship 2024Posted 12 minutes ago
Can I get an A/A* using claire bio and ms estruch vids for OCR A level biologyLast reply 12 minutes ago
Government Social Research - Research Officer Scheme 2024Trending
Last reply 3 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]Trending
Last reply 3 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]


