Position of Equilibrium help
Hi guys, a bit stuck on this question. Can anyone please explain it to me?

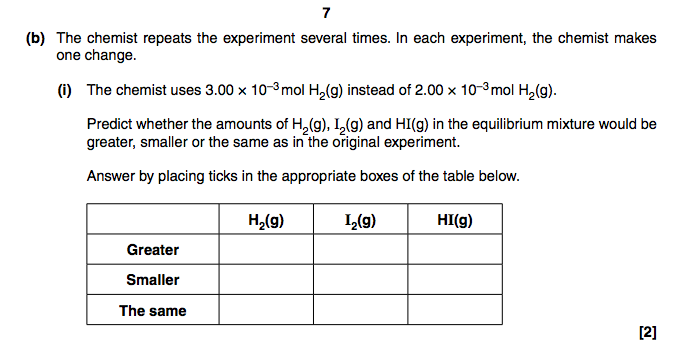
The answer for the first part is 16.8(no units) which I got right. I just can't seem to understand the second part.


The answer for the first part is 16.8(no units) which I got right. I just can't seem to understand the second part.
Original post by TheNoobishKnight
Hi guys, a bit stuck on this question. Can anyone please explain it to me?


The answer for the first part is 16.8(no units) which I got right. I just can't seem to understand the second part.


The answer for the first part is 16.8(no units) which I got right. I just can't seem to understand the second part.
Use the equilibrium constant from part (a) and the equilibrium law equation to estimate the values at equilibrium of the components.
kc = [HI]2 / [H2][I2]
and you know that x moles of H2 and I2 have reacted at equilibrium and formed 2x moles of HI
... but the simple rule is: if you add to the left you make more of the right ...
(edited 9 years ago)
Original post by charco
Use the equilibrium constant from part (a) and the equilibrium law equation to estimate the values at equilibrium of the components.
kc = [HI]2 / [H2][I2]
and you know that x moles of H2 and I2 have reacted at equilibrium and formed 2x moles of HI
... but the simple rule is: if you add to the left you make more of the right ...
kc = [HI]2 / [H2][I2]
and you know that x moles of H2 and I2 have reacted at equilibrium and formed 2x moles of HI
... but the simple rule is: if you add to the left you make more of the right ...
Yup that's what I thought. More H2 is added, then more Hi needs to be made to make up for it. But I couldn't understand what happened to I2?
Original post by Borek
Iodine is consumed in the reaction till reaction quotient equals the equilibrium constant. It won't ever got down to zero.
Okay. I am still a bit confused about what happens to I2, especially in different scenarios but I will ask my teacher. Thanks for the help buddy

Like you add more to left, get more on right. But why is I2 being used up when more H2 is added, isn't meant to stay the same to further reduce more shifting of the Position of Equilibrium?
(edited 9 years ago)
Original post by TheNoobishKnight
Okay. I am still a bit confused about what happens to I2, especially in different scenarios but I will ask my teacher. Thanks for the help buddy 
Like you add more to left, get more on right. But why is I2 being used up when more H2 is added, isn't meant to stay the same to further reduce more shifting of the Position of Equilibrium?

Like you add more to left, get more on right. But why is I2 being used up when more H2 is added, isn't meant to stay the same to further reduce more shifting of the Position of Equilibrium?
It has no choice!
If you add hydrogen the equilibrium is disturbed and the system must respond by moving to the right hand side.
It can only do this by reacting hydrogen and iodine together. The hydrogen needs the iodine to react with...
Hence, increase the hydrogen and the system reacts hydrogen and iodine together to make more hydrogen iodide and restore the equilibrium situation.
Original post by charco
It has no choice!
If you add hydrogen the equilibrium is disturbed and the system must respond by moving to the right hand side.
It can only do this by reacting hydrogen and iodine together. The hydrogen needs the iodine to react with...
Hence, increase the hydrogen and the system reacts hydrogen and iodine together to make more hydrogen iodide and restore the equilibrium situation.
If you add hydrogen the equilibrium is disturbed and the system must respond by moving to the right hand side.
It can only do this by reacting hydrogen and iodine together. The hydrogen needs the iodine to react with...
Hence, increase the hydrogen and the system reacts hydrogen and iodine together to make more hydrogen iodide and restore the equilibrium situation.
OHHHHHHHHHHH NOW I get it.
A needs to make C but to do that it needs to B. So there is more A(added), less B and more C(to reduce the effect of the increase of the left side).
You rock

Original post by TheNoobishKnight
OHHHHHHHHHHH NOW I get it.
A needs to make C but to do that it needs to B. So there is more A(added), less B and more C(to reduce the effect of the increase of the left side).
You rock
A needs to make C but to do that it needs to B. So there is more A(added), less B and more C(to reduce the effect of the increase of the left side).
You rock

Not so hard once you see the light

Original post by charco
Not so hard once you see the light 

My fav thing about Chemistry
 . The feeling after understanding something
. The feeling after understanding something 
Quick Reply
Related discussions
- GCSE Chemistry Triple Science
- What affects Kp?
- le chapeliers principle, exo and endo?????
- Maths Further ( Mechanics)
- Confused about Kp and Kc - help please
- Kw Question A Level Chem
- The Weighing Machine - Isaac physics
- More questions like this waves in water problem?
- The eqm constant question
- AQA A Level Physics Waves Question
- AQA GCSE Combined Science Paper 2 Higher Tier (8464/C/2H) -13th June 2023 [Exam Chat]
- A level physics SHM mcq help
- Moments
- A level chemistry ocr
- Chem equilibrium
- Chemistry Kc Question
- NEED HELP with KC equilibrium question please!
- Equilibrium
- Is my Head Prefect application okay? (sixth form)
- please help chem question!!
Latest
Last reply 1 minute ago
Amazon Project management apprenticeship 2024Last reply 1 minute ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 5 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 6 minutes ago
Official: University of St Andrews A100 Offer Holders 2024 entryLast reply 12 minutes ago
Why is the political left now censorious and authoritarian??Last reply 12 minutes ago
Is anyone else more aroused/turned on when having to pee really badly?Last reply 13 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 13 minutes ago
LSE Economic history departmentLast reply 13 minutes ago
National Probation ServiceLast reply 14 minutes ago
Best university to study Film studies/production?Last reply 15 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2916
Last reply 15 minutes ago
National Audit Office Apprenticeship 2024Trending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products


