RE: Organic Chemistry
Hello all,
I am currently doing my AS chemistry and am struggling with the Organic section of it!
What I don't understand is what the brackets mean and also, a few specific structural formulae that I don't understand the displayed formula's of:
(C2H5)2CHCH(CH3)CH(CH3)2
Also, I have been given the structural formula but it looks more like a displayed formula as it has the double bonds and single bonds.
CH3-CH2-CH=CH2 what would the displayed formula for that be?
Also, I don't understand how to draw the skeletal formula as well?
Sorry, I know there are a lot of problems- but, I'll be giving reps for helping out!
Thanks!
I am currently doing my AS chemistry and am struggling with the Organic section of it!
What I don't understand is what the brackets mean and also, a few specific structural formulae that I don't understand the displayed formula's of:
(C2H5)2CHCH(CH3)CH(CH3)2
Also, I have been given the structural formula but it looks more like a displayed formula as it has the double bonds and single bonds.
CH3-CH2-CH=CH2 what would the displayed formula for that be?
Also, I don't understand how to draw the skeletal formula as well?
Sorry, I know there are a lot of problems- but, I'll be giving reps for helping out!
Thanks!
Original post by science_geeks
Hello all,
I am currently doing my AS chemistry and am struggling with the Organic section of it!
What I don't understand is what the brackets mean and also, a few specific structural formulae that I don't understand the displayed formula's of:
(C2H5)2CHCH(CH3)CH(CH3)2
Also, I have been given the structural formula but it looks more like a displayed formula as it has the double bonds and single bonds.
CH3-CH2-CH=CH2 what would the displayed formula for that be?
Also, I don't understand how to draw the skeletal formula as well?
Sorry, I know there are a lot of problems- but, I'll be giving reps for helping out!
Thanks!
I am currently doing my AS chemistry and am struggling with the Organic section of it!
What I don't understand is what the brackets mean and also, a few specific structural formulae that I don't understand the displayed formula's of:
(C2H5)2CHCH(CH3)CH(CH3)2
Also, I have been given the structural formula but it looks more like a displayed formula as it has the double bonds and single bonds.
CH3-CH2-CH=CH2 what would the displayed formula for that be?
Also, I don't understand how to draw the skeletal formula as well?
Sorry, I know there are a lot of problems- but, I'll be giving reps for helping out!
Thanks!
The brackets mean two things. In a formula, they can be used to determine how many of a polyatomic is there.
For instance, if you write Magnesium Hydroxide, you write Mg(OH)2.
Not MgO2H2 because the {OH-} is the structure that bonds to Magnesium.
The brackets also help to indicate what is bonded to what.
For instance, CH3CH2CH3 is just propane.
But say I stick a methyl group on the second carbon, i.e. methylpropane
I have to write it as CH3CH(CH3)CH3 and the brackets show that the methyl group is branching off that second carbon (if you're reading left to right).
If I instead wrote it as CH3CHCH3CH3 then it might be confusing as it appears to be butane. Of course, upon closer inspection we can see that it's wrong seeing as one carbon is making 3 bonds and another is making 5 bonds.
In this case: (C2H5)2CHCH(CH3)CH(CH3)2
The first carbon has two ethyl groups branching off it (and a hydrogen of course). The next carbon has a methyl group branching off it. The third carbon has two methyl groups branching off the end.
It helps to draw it out to visualise it, but the resulting molecule would be called:
4-ethyl-2,3-dimethylhexane (I hope)
NB: We draw double bonds in the structural formula to avoid any ambiguity.
Displayed structural formula by the way is drawing absolutely every single bond in the molecule.
Skeletal formula is like drawing the displayed formula, except you do not draw the hydrogen atoms and the number of carbon atoms is equal to the number of vertices.
For instance, take propane again.
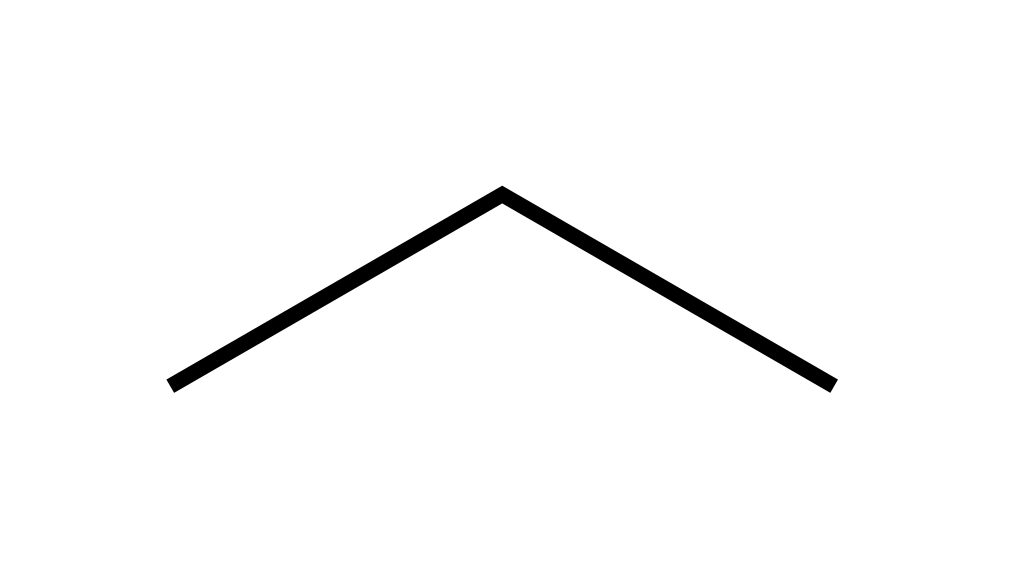
It's not drawn as a straight line, otherwise how else could I know how long the molecule is. Instead, from one carbon to the next is a straight line. But then to the carbon after that, you draw a line at an angle. Think of the lines as bonds and place a carbon atom at the end of each line segment. Notice that no hydrogen atoms are shown. If I wanted to draw methylpropane, I would simply draw a line upwards at the second carbon (i.e. the pointy bit)
Original post by RMNDK
The brackets mean two things. In a formula, they can be used to determine how many of a polyatomic is there.
For instance, if you write Magnesium Hydroxide, you write Mg(OH)2.
Not MgO2H2 because the {OH-} is the structure that bonds to Magnesium.
The brackets also help to indicate what is bonded to what.
For instance, CH3CH2CH3 is just propane.
But say I stick a methyl group on the second carbon, i.e. methylpropane
I have to write it as CH3CH(CH3)CH3 and the brackets show that the methyl group is branching off that second carbon (if you're reading left to right).
If I instead wrote it as CH3CHCH3CH3 then it might be confusing as it appears to be butane. Of course, upon closer inspection we can see that it's wrong seeing as one carbon is making 3 bonds and another is making 5 bonds.
In this case: (C2H5)2CHCH(CH3)CH(CH3)2
The first carbon has two ethyl groups branching off it (and a hydrogen of course). The next carbon has a methyl group branching off it. The third carbon has two methyl groups branching off the end.
It helps to draw it out to visualise it, but the resulting molecule would be called:
4-ethyl-2,3-dimethylhexane (I hope)
NB: We draw double bonds in the structural formula to avoid any ambiguity.
Displayed structural formula by the way is drawing absolutely every single bond in the molecule.
Skeletal formula is like drawing the displayed formula, except you do not draw the hydrogen atoms and the number of carbon atoms is equal to the number of vertices.
For instance, take propane again.

It's not drawn as a straight line, otherwise how else could I know how long the molecule is. Instead, from one carbon to the next is a straight line. But then to the carbon after that, you draw a line at an angle. Think of the lines as bonds and place a carbon atom at the end of each line segment. Notice that no hydrogen atoms are shown. If I wanted to draw methylpropane, I would simply draw a line upwards at the second carbon (i.e. the pointy bit)
For instance, if you write Magnesium Hydroxide, you write Mg(OH)2.
Not MgO2H2 because the {OH-} is the structure that bonds to Magnesium.
The brackets also help to indicate what is bonded to what.
For instance, CH3CH2CH3 is just propane.
But say I stick a methyl group on the second carbon, i.e. methylpropane
I have to write it as CH3CH(CH3)CH3 and the brackets show that the methyl group is branching off that second carbon (if you're reading left to right).
If I instead wrote it as CH3CHCH3CH3 then it might be confusing as it appears to be butane. Of course, upon closer inspection we can see that it's wrong seeing as one carbon is making 3 bonds and another is making 5 bonds.
In this case: (C2H5)2CHCH(CH3)CH(CH3)2
The first carbon has two ethyl groups branching off it (and a hydrogen of course). The next carbon has a methyl group branching off it. The third carbon has two methyl groups branching off the end.
It helps to draw it out to visualise it, but the resulting molecule would be called:
4-ethyl-2,3-dimethylhexane (I hope)
NB: We draw double bonds in the structural formula to avoid any ambiguity.
Displayed structural formula by the way is drawing absolutely every single bond in the molecule.
Skeletal formula is like drawing the displayed formula, except you do not draw the hydrogen atoms and the number of carbon atoms is equal to the number of vertices.
For instance, take propane again.

It's not drawn as a straight line, otherwise how else could I know how long the molecule is. Instead, from one carbon to the next is a straight line. But then to the carbon after that, you draw a line at an angle. Think of the lines as bonds and place a carbon atom at the end of each line segment. Notice that no hydrogen atoms are shown. If I wanted to draw methylpropane, I would simply draw a line upwards at the second carbon (i.e. the pointy bit)
Thank you, that makes a lot more sense!

Quick Reply
Related discussions
- Learning techniques
- I have been struggling with my a levels, how do I improve?
- Oxford chemistry
- Economics at Trinity Cambridge
- Personal statement help for chemistry
- OCR A-Level Chemistry B Paper 2 (H433/02) - 19th June 2023 [Exam Chat]
- Chemistry A level Help!
- Organic chemistry textbook (uni)
- aqa a level chemistry paper 2 assessed topics
- Chemistry at Uni?
- physical or inorganic??
- How can I become obsessed with chemistry ??
- Chemistry revision
- The A-level grind
- A level chemistry for a biology degree? (help please!!)
- Tips for physical A level chemistry OCR A?
- UCL Natural Sciences and streams
- Advice for getting A*s in A levels
- Is Chemistry better at A-level than at GCSE?
- AS/A Level Chemistry Study Group 2023/2024
Latest
Last reply 3 minutes ago
I got accepted into King's for CS, is waiting for UCL UPC's response worth it?Last reply 9 minutes ago
Official MMU Offer Holders Thread for 2024 entryLast reply 11 minutes ago
student finance and nhs fund for 2nd healthcare degree?Last reply 17 minutes ago
HSBC Degree Apprenticeship 2024Last reply 18 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 21 minutes ago
PWC Flying Start Degree Apprenticeships 2024!Trending
Last reply 3 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]Trending
Last reply 3 days ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]


