NMR Spectroscopy help, Alevel Chem
For each structure, predict the number of peaks in its low resolution nmr spectrum corresponding to the different types of proton and also the number of each different type of proton (e.g. CH3CH2OH has 3 peaks in ratio 3:2:1).
(a) CH3OH (b)CH3CH2CHO (c) CH3COCH3 (d) (CH3) 2CHOH
(a) CH3OH (b)CH3CH2CHO (c) CH3COCH3 (d) (CH3) 2CHOH
Low resolution means you don't have to worry about splitting due to protons in adjacent environments (if you haven't learnt about that yet, not a problem here since we are ignoring it).
The most helpful thing you can do in this question is to start by drawing out each structure.
For (a) this would be
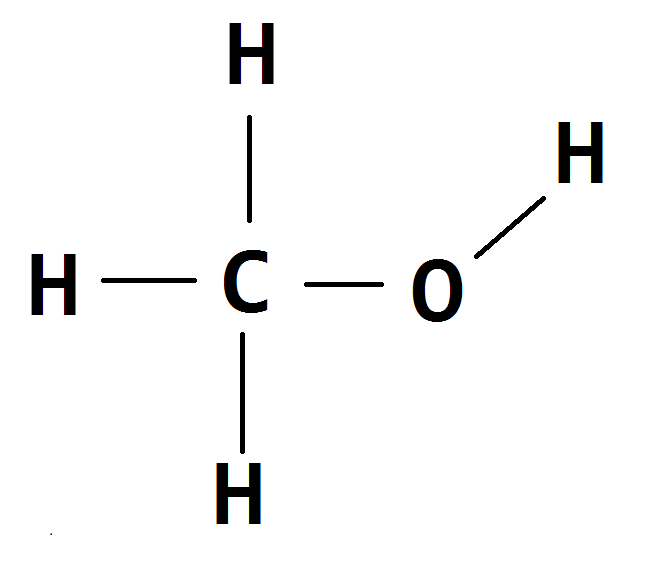
You then need to identify which protons are in different environments. Here there are only 2 environments: the 3 H bonded to the C and the 1 H bonded to the O. Each of these different environments leads to 1 peak on the NMR spectrum, and the number of hydrogens relates to the size of the peak.
The most helpful thing you can do in this question is to start by drawing out each structure.
For (a) this would be

You then need to identify which protons are in different environments. Here there are only 2 environments: the 3 H bonded to the C and the 1 H bonded to the O. Each of these different environments leads to 1 peak on the NMR spectrum, and the number of hydrogens relates to the size of the peak.
Quick Reply
Related discussions
- better late than never - gyg 24' :)
- The A-level grind
- Grade Growth Chronicles | From C's to A's (23-24)
- Help spectroscopy
- Spectroscopy help
- Help urgent spectroscopy
- Jieay's Year 13 GYG - Actuarial Science applicant (2023/2024)
- MY GYG
- AS chemistry paper 2 2022 AQ
- Chemistry at Uni?
- How do you do NMR questions!?
- Bad predicted grades in year 12
- AQA Alevel Chemistry NMR
- Physics vs further maths difficulty
- What should i pick?
- Chemistry
- (help pleaseee) A Level Options for Medicine
- Should I take chemistry or english lit?
- YEAR 13 study buddy
- Holding myself accountable; study!
Latest
Trending
Last reply 9 hours ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10
Trending
Last reply 9 hours ago
Edexcel A Level Politics Paper 1 (9PL0 01) - 21st May 2024 [Exam Chat]A-levels
10




