This discussion is now closed.
Check out other Related discussions
- Kc Titration Question
- Titration paper
- Etha
- Titration and Moles question
- hard titration alevel chem Q!
- A level Chemistry Titration
- Chem alevel help
- Titration questions
- Chemistry Calculation Question
- Chemistry calculation help please!
- Btec applied science unit 2
- chemistry titration
- [URGENT] stuck on titration a level chemistry question
- chem help
- Back-titration question
- As level / a level chemistry concentration help!!
- AS Titrations?
- gcse predictions
- Hard A Level Chemistry Question
- chem a level question
Titration Calculations Question HELP
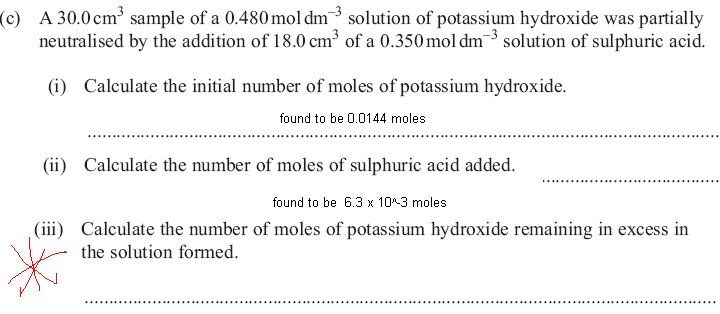
On the mark scheme, for iii, it says:
Why x2 moles?? Can someone help me with the equation itself so I can see the mole ratio- I've tried for ages but I'm rubbish at balancing equations and stuff D:
malolis
KOH reacts in a 2:1 ratio to sulphuric acid.
Sulphuric acid - H2SO4 and therefore diprotic - dissociates into 2 H ions for every molecule of H2SO4 so, each molecule of H2SO4 reacts with 2 molecules of KOH.
Sulphuric acid - H2SO4 and therefore diprotic - dissociates into 2 H ions for every molecule of H2SO4 so, each molecule of H2SO4 reacts with 2 molecules of KOH.
OHHHHHHHHHHHH
I forgot about the whole H+ business >____>
Btw, is KHSO4 valid?
H2SO4 dissociates in two stages:
H2SO4 ---> H+ + HSO4-
HSO4- ---> H+ + SO4-
H2SO4 ---> H+ + HSO4-
HSO4- ---> H+ + SO4-
malolis
Yes but he's not really required to know that at A level and it doesn't really affect his answer. ^^
I didn't want to confuse him
I didn't want to confuse him

Okay...but he's a she by the way

Related discussions
- Kc Titration Question
- Titration paper
- Etha
- Titration and Moles question
- hard titration alevel chem Q!
- A level Chemistry Titration
- Chem alevel help
- Titration questions
- Chemistry Calculation Question
- Chemistry calculation help please!
- Btec applied science unit 2
- chemistry titration
- [URGENT] stuck on titration a level chemistry question
- chem help
- Back-titration question
- As level / a level chemistry concentration help!!
- AS Titrations?
- gcse predictions
- Hard A Level Chemistry Question
- chem a level question
Latest
Last reply 2 minutes ago
student finance and nhs fund for 2nd healthcare degree?Last reply 8 minutes ago
HSBC Degree Apprenticeship 2024Last reply 10 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 12 minutes ago
PWC Flying Start Degree Apprenticeships 2024!Last reply 13 minutes ago
Google level 4 software development apprenticeshipLast reply 15 minutes ago
Accenture Degree Apprenticeship 2024Last reply 17 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Last reply 17 minutes ago
Official: University of Cardiff A100 2024 Entry ApplicantsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these products




