Naming things with: Methyl and Ethyl whats the difference?
ok, so when naming hydrocarbons, how do you know when to put methyl and when to put ethyl?
I've been told that ethyl is one CH3 group...
what do u put when there are two CH3's coming off one C atom, but on different 'sides'.
and what do you put when there are two CH3's joined together coming off a C atom??
thanks
I've been told that ethyl is one CH3 group...
what do u put when there are two CH3's coming off one C atom, but on different 'sides'.
and what do you put when there are two CH3's joined together coming off a C atom??
thanks
Scroll to see replies
Methyl=1 carbon chain branched off the parent alkane
Ethyl = 2 carbon chain branched off the parent alkane
If 2 methyl groups are presnet on different positions its di-methyl
Ethyl = 2 carbon chain branched off the parent alkane
If 2 methyl groups are presnet on different positions its di-methyl
Holdo24
methy= 1 ch3 group
dimethyl= 2 ch3 groups on same C
ethyl= c2h5 group
methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl
Am I right?
dimethyl= 2 ch3 groups on same C
ethyl= c2h5 group
methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl
Am I right?
Yes
raspberryice
ok, so when naming hydrocarbons, how do you know when to put methyl and when to put ethyl?
I've been told that ethyl is one CH3 group...
what do u put when there are two CH3's coming off one C atom, but on different 'sides'.
and what do you put when there are two CH3's joined together coming off a C atom??
thanks
I've been told that ethyl is one CH3 group...
what do u put when there are two CH3's coming off one C atom, but on different 'sides'.
and what do you put when there are two CH3's joined together coming off a C atom??
thanks
Methyl is just one CH3 group.
Ethyl is a -CH2-CH3 group.
If you have 2 CH3s coming off one carbon (ie 2 methyl groups) you number the carbon and then use that number twice, for example
2,2-dimethylhexane.
Would have 2 methyl groups on the second carbon along.
"and what do you put when there are two CH3's joined together coming off a C atom??"
I'm not sure what you mean by that?
Holdo24 Thankyou. (crashing Higher chemistry this year)
Thankyou. (crashing Higher chemistry this year)
 Thankyou. (crashing Higher chemistry this year)
Thankyou. (crashing Higher chemistry this year)Hehe
I'm failing A2 chemistry this year

OP what you mean by "and what do you put when there are two CH3's joined together coming off a C atom??"??
Please expand
Loz17
Hehe
I'm failing A2 chemistry this year
OP what you mean by "and what do you put when there are two CH3's joined together coming off a C atom??"??
Please expand
I'm failing A2 chemistry this year

OP what you mean by "and what do you put when there are two CH3's joined together coming off a C atom??"??
Please expand
I meant 1 CH2, then CH3 in one branch. (attached to each other) sorry.
raspberryice
I meant 1 CH2, then CH3 in one branch. (attached to each other) sorry.
= Ethyl group
= Methyl group
Adhavan
= Ethyl group
= Methyl group
= Methyl group
thanks....i'm nearly there...
soo
what is this?
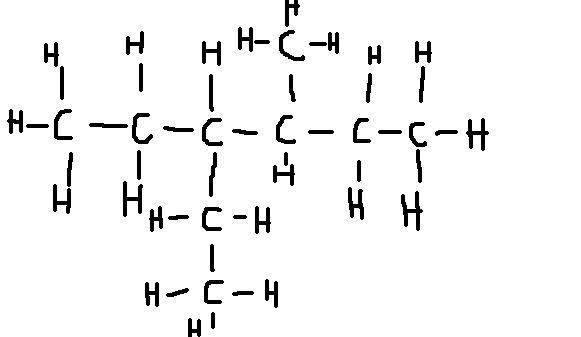
4-ethyl 3-methylhexane??
raspberryice
4-ethyl 3-methylhexane??
Nearly: 3-ethyl-4-methylhexane
raspberryice
thanks....i'm nearly there...
soo
what is this?

4-ethyl 3-methylhexane??
soo
what is this?

4-ethyl 3-methylhexane??

or 3-ethyl4-methylhexane (i think you're supposed to list them in alphabetical/number order but i don't think it matters that much at this stage)
EierVonSatan
Nearly: 3-ethyl-4-methylhexane
ah right thanks, so you go from the other end, because the ethyl is longer?
also, when u hav a cyclic hydrocarbon, and only one ethyl (CH2-CH3) thingy coming off it, how do u number it?
thanks a bunch everyone!
raspberryice
ah right thanks, so you go from the other end, because the ethyl is longer?
No it's because it has to be alphabetical order ethyl before methyl
also, when u hav a cyclic hydrocarbon, and only one ethyl (CH2-CH3) thingy coming off it, how do u number it?
If there is only one, it doesn't need a number

EierVonSatan
No it's because it has to be alphabetical order ethyl before methyl
If there is only one, it doesn't need a number
If there is only one, it doesn't need a number

thank uuu

Something strange about chem..it can b hard at times, but i still enjoy it!
weird...
raspberryice
ok..
thank uuu
Something strange about chem..it can b hard at times, but i still enjoy it!
weird...
thank uuu

Something strange about chem..it can b hard at times, but i still enjoy it!
weird...
yeah, I have the same thoughts now

EierVonSatan
yeah, I have the same thoughts now 

eggs of...... satan......
this one is also an egg of satan
Oracle_163

Oracle_163
urr...if u say so.

Quick Reply
Related discussions
- Chemistry naming alkanes draw structure
- AQA Chemistry Alevel Organic synthesis
- Esterification
- A level Chemistry OCR HELP
- A level chemistry optical isomerism MC questions
- Spectroscopy help
- chem isomer as help?
- how is ethyl ethanoate manufactured industrially
- Boiling point of halogenoalkanes
- A level Chemistry help
- Skeletal Formulae
- Applied Science Unit 4
- Why is1-methylpentane not an isomer of Hexane
- Chemistry question help
- is but-1-ene the same as butene
- Amount of substance chemistry
- How come this isn't written as 2,2-dimethyl-4-chlorobutanal
- Nucleophilic substitution - Need help
- Help chemistry
- kinetics
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



