This discussion is now closed.
Check out other Related discussions
- Will the bond angle in H2O(104.5) increase if water is bind to other molecules?
- a level chemistry aqa
- shape of molecules a level chem
- factors effecting bond strength
- a level chemistry drawing moelceules
- Learndirect- presentation advice
- Shapes of molecules and their names
- chemistry shapea of molecules a level
- AS/A Level Chemistry Study Group 2023/2024
- Shapes of molecules
- a level chemistry
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- chemistry question
- Bad interview-pharmacy
- Maths Problem Please Help
- Physics help
- Maths nerds!
- Snell’s Law AQA A-Level Physics
- Bond breaking and intermolecular force A level
- Physics question circular motion
bond angles
hi
basically i know all the bond angles but am confused when they say work out bond angle for COH or something...which bond do i look at?
basically i know all the bond angles but am confused when they say work out bond angle for COH or something...which bond do i look at?
I always think, if there are 4 bonded pairs with no lone pairs then the angle is 109deg. Then for every bonded pair you take off and lone pair you put on you take off 2.5deg. So water with two bonded pairs and two lone pairs has a bond angle of 104deg.
If that makes sense
If that makes sense

El-Taji
hi
basically i know all the bond angles but am confused when they say work out bond angle for COH or something...which bond do i look at?
basically i know all the bond angles but am confused when they say work out bond angle for COH or something...which bond do i look at?
Unless I've missed something, COH couldn't exist as a molecule: it would need an extra hydrogen atom and be CHOH - methanal.
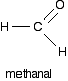
Since methanal looks like that, the bond angle is invariable as there are three regions of electron density. Therefore the overall bond shape is trigonal planar and the bond angle is consequently 120 degrees.
Hope this helps - sorry if I've got the wrong end of the stick!
By COH they mean the angle between the C-O bond and the O-H bond, which would be similar to the bond angle in water (104.5o) 

Related discussions
- Will the bond angle in H2O(104.5) increase if water is bind to other molecules?
- a level chemistry aqa
- shape of molecules a level chem
- factors effecting bond strength
- a level chemistry drawing moelceules
- Learndirect- presentation advice
- Shapes of molecules and their names
- chemistry shapea of molecules a level
- AS/A Level Chemistry Study Group 2023/2024
- Shapes of molecules
- a level chemistry
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- chemistry question
- Bad interview-pharmacy
- Maths Problem Please Help
- Physics help
- Maths nerds!
- Snell’s Law AQA A-Level Physics
- Bond breaking and intermolecular force A level
- Physics question circular motion
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



