Organic Chem question.
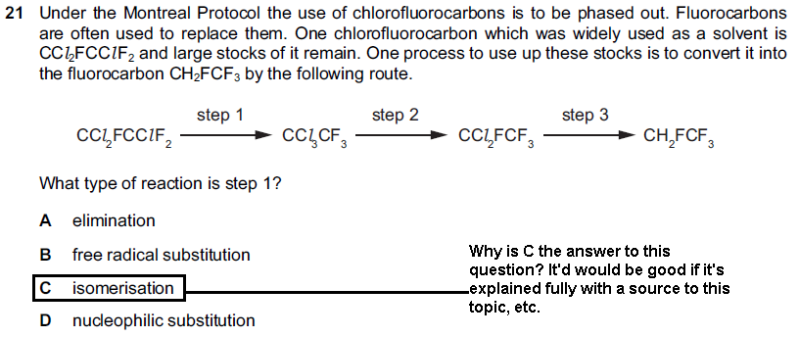
What type of reaction is isomerisation? Neither my syllabus states anything about it nor any text book of A level tells that isomerisation is any kind of reaction! But the past papers gives this question and the mark scheme states its answer as C, i.e Isomerisation...
(edited 13 years ago)
If two different compounds have the same molecular formula, then they are isomers of each other 

Original post by EierVonSatan
If two different compounds have the same molecular formula, then they are isomers of each other 

Original post by mobius323
Looks to me as if the fluorine on the 1st Carbon has swapped with the chlorine on the 2nd Carbon.
The molecular formula of the molecule will not have changed. The structural formula has. Isomers are molecules with the same molecular formulae but different structural formulae.
The molecular formula of the molecule will not have changed. The structural formula has. Isomers are molecules with the same molecular formulae but different structural formulae.
Oh, wow. Thanks - I didn't even notice that. Btw isomerisation isn't any TYPE of reaction, right?
Original post by Zishi
Oh, wow. Thanks - I didn't even notice that. Btw isomerisation isn't any TYPE of reaction, right?
well bonds are broken and bonds are formed, so i would consider it to be so.
Original post by shengoc
well bonds are broken and bonds are formed, so i would consider it to be so.
Hmm, thanks. I got it.

Original post by Zishi
Oh, wow. Thanks - I didn't even notice that. Btw isomerisation isn't any TYPE of reaction, right?
It's a tricky one, because (and this is just a hunch, but it makes sense), in the reaction above for example, a C-F bond is broken and a C-Cl bond is broken. Now after that, a C-Cl bond is made in place of the broken C-F bond, and a C-F bond is made in place of the C-Cl bond.
I'd say it's a reaction, yeah.
(edited 13 years ago)
Original post by mobius323
It's a tricky one, because (and this is just a hunch, but it makes sense), in the reaction above for example, a C-F bond is broken and a C-Cl bond is broken. Now after that, a C-Cl bond is made in place of the broken C-F bond, and a C-F bond is made in place of the C-Cl bond.
Now, going back to the dreaded Energetics (more precisely, bond enthalpies), you should know that making bonds is exactly as exothermic and breaking bonds is endothermic. This meant that the Enthalpy Change of Reaction would be zero. This would mean total energy in = total energy out.
I'd say it's a reaction, yeah.
Now, going back to the dreaded Energetics (more precisely, bond enthalpies), you should know that making bonds is exactly as exothermic and breaking bonds is endothermic. This meant that the Enthalpy Change of Reaction would be zero. This would mean total energy in = total energy out.
I'd say it's a reaction, yeah.
I don't think the assumption is neccesaily true. Diffferent groups bonded to a C atom will change the bond energy of other groups attached to the same C atom. For example, the C-H bond energy in choloromethane is different to the C-H bond in methane.
(edited 13 years ago)
Original post by Maker
I don't think the assumption is neccesaily true. Diffferent groups bonded to a C atom will change the bond energy of other groups attached to the same C atom. For example, the C-H bond energy in choloromethane is different to the C-H bond in methane.
Hmmm, perhaps. If you model each Carbon as a seperate molecule, then I can see what you're talking about. I can see how the polarisation of the Carbon atoms would be different.
As said above, the molecular formula is the same for each compound. They are structural isomers of each other. I don't think the enthalpy change of the reaction will be zero either because the environment of the C-Cl bond in C-Cl3-C and CCl2F-C are different which would effect how the carbon atom attracts electrons and therefore the enthalpy of the bond
Quick Reply
Related discussions
- organic chem - a level ocr
- A-level chem help
- Tips for physical A level chemistry OCR A?
- Mpharm Nottingham
- year 12 study journal!
- should i give up?
- Chem alevel
- Please help me how do i learn most of chemistry for alevel in less than two weeks
- Grade Growth Chronicles | From C's to A's (23-24)
- I have been struggling with my a levels, how do I improve?
- kingston mpharm 2024
- A level chemistry for a biology degree? (help please!!)
- chemistry alevel
- HELP im horrible at module 2 OCR chemistry
- chemistry help
- Natural Sciences at UCL or Bath?
- A-level choices
- my question is about a level
- Any last minute A level tips?
- Chemistry or Biochemistry?
Latest
Last reply 1 minute ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 5 minutes ago
NICS Staff Officer and Deputy Principal recruitment 2022 2023Last reply 11 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1292
Last reply 11 minutes ago
The Official King's College London Applicants for 2024 Entry ThreadLast reply 14 minutes ago
Woodhouse College applicants 2024Last reply 15 minutes ago
BAE systems degree apprenticeships September 2024Last reply 16 minutes ago
OCR A-LEVEL PSYCHOLOGY PAPER 3 (H567/03) - 3rd June [Exam Chat]Last reply 18 minutes ago
OCR A-LEVEL PSYCHOLOGY PAPER 2 (H567/02) - 22nd May [Exam Chat]Last reply 19 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 21 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 26 minutes ago
Are there any good sixth forms/sixth form colleges with applications still open?Last reply 27 minutes ago
Feeling inferior compared to syrians as a half moroccan/half whiteLast reply 27 minutes ago
Telecommunications engineer job with "Provisional" licence?Last reply 28 minutes ago
Edexcel A-level French Paper 3, IRP/Speaking (9FR0 03) - 2024 [Exam Chat]



