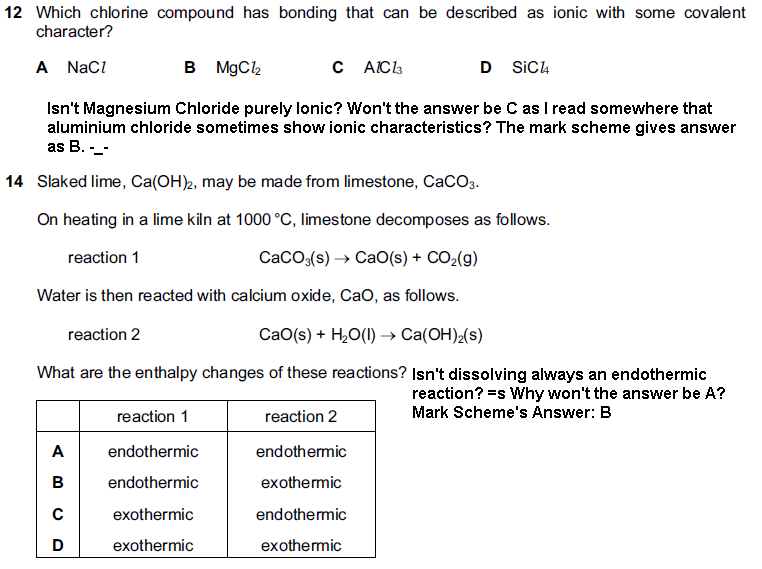
12 no, MgCl2 is not purely ionic. It has some covalent character (Mg is a small ion with a relatively high charge, therefore polarizing)
14 What has dissolving got to do with anything? The reaction 2 is strongly exothermic as the CaO reacts with the water
17 sulphur burns in excess oxygen to form SO2. It can't be oxidised further without catalytic help
37 burning is exothermic, yes. But that's not the question. They are asking why nitrogen and oxygen don't react at RT
14 What has dissolving got to do with anything? The reaction 2 is strongly exothermic as the CaO reacts with the water
17 sulphur burns in excess oxygen to form SO2. It can't be oxidised further without catalytic help
37 burning is exothermic, yes. But that's not the question. They are asking why nitrogen and oxygen don't react at RT
(edited 13 years ago)
Original post by Plato's Trousers
A no, MgCl2 is not purely ionic. It has some covalent character (Mg is a small ion with a relatively high charge, therefore polarizing)
B What has dissolving got to do with anything? The reaction 2 is strongly exothermic as the CaO reacts with the water
I was taking the example of Ammonium Chloride with water - it is an endothermic reaction and has many commercial uses. So won't Calcium Oxide be dissolving, too?

C sulphur burns in excess oxygen to form SO2. It can't be oxidised further without catalytic help
Oh, I didn't notice that.

D burning is exothermic, yes. But that's not the question. They are asking why nitrogen and oxygen don't react at RT
But even if it has to do with 'why they don't react', the statement should also be correct. If burning is always exothermic, then statement 1 is wrong.

Original post by Zishi
Oh, okay!
I was taking the example of Ammonium Chloride with water - it is an endothermic reaction and has many commercial uses. So won't Calcium Oxide be dissolving, too?
Oh, I didn't notice that.
But even if it has to do with 'why they don't react', the statement should also be correct. If burning is always exothermic, then statement 1 is wrong.
I was taking the example of Ammonium Chloride with water - it is an endothermic reaction and has many commercial uses. So won't Calcium Oxide be dissolving, too?

Oh, I didn't notice that.

But even if it has to do with 'why they don't react', the statement should also be correct. If burning is always exothermic, then statement 1 is wrong.

Mind you for the last bit, i believe chemistry books don't define the reaction "burning"
and combustion is the formal word. and yes, this is a question of whether is it spontaneous process and if it is, is it fast?
Original post by shengoc
Mind you for the last bit, i believe chemistry books don't define the reaction "burning"
and combustion is the formal word. and yes, this is a question of whether is it spontaneous process and if it is, is it fast?
and combustion is the formal word. and yes, this is a question of whether is it spontaneous process and if it is, is it fast?
But isn't reaction with oxygen aka burning?

Original post by Zishi
But isn't reaction with oxygen aka burning? 

it is, but would you have seen standard enthalpy of burning in standard texts?
Original post by Zishi
But isn't reaction with oxygen aka burning? 

yes, but that is irrelevant. They are asking why it doesn't happen at RT, not whether it's endo or exo thermic. Whether something is endo/exo thermic only tells you the delta H of the reaction. It says nothing about whether it will spontaneously occur.
Original post by shengoc
it is, but would you have seen standard enthalpy of burning in standard texts?
It is the enthalpy change when one mole of an element OR compound burns completely in air to give the products in their standard stats under standard conditions. I just can't understand that even you're agreeing that burning is exothermic than why is STILL statement number one correct? Even if it has nothing to do with the reaction at RT. It's wrong at first place.

Original post by Plato's Trousers
yes, but that is irrelevant. They are asking why it doesn't happen at RT, not whether it's endo or exo thermic. Whether something is endo/exo thermic only tells you the delta H of the reaction. It says nothing about whether it will spontaneously occur.
So do you mean that even if the first statement is literally wrong, we just have to answer the question in the sense in which it's being asked?

Original post by Zishi
It is the enthalpy change when one mole of an element OR compound burns completely in air to give the products in their standard stats under standard conditions. I just can't understand that even you're agreeing that burning is exothermic than why is STILL statement number one correct? Even if it has nothing to do with the reaction at RT. It's wrong at first place. 
So do you mean that even if the first statement is literally wrong, we just have to answer the question in the sense in which it's being asked?

So do you mean that even if the first statement is literally wrong, we just have to answer the question in the sense in which it's being asked?

It's a multi-choice question ffs! Not all the answers are correct (that's the whole point!). You have to pick the right one!
Example:
What is a dog?
A a four legged mammal
B a glass measuring instrument
C a country in Asia
(clue : only one of those is correct)
(edited 13 years ago)
Original post by Plato's Trousers
It's a multi-choice question ffs! Not all the answers are correct (that's the whole point!). You have to pick the right one!
Example:
What is a dog?
A a four legged mammal
B a glass measuring instrument
C a country in Asia
(clue : only one of those is correct)
Example:
What is a dog?
A a four legged mammal
B a glass measuring instrument
C a country in Asia
(clue : only one of those is correct)
Sorry for not mentioning about it before but it's a three-statement multiple completion item.
Here's the heading for this question:
Original post by Zishi
It is the enthalpy change when one mole of an element OR compound burns completely in air to give the products in their standard stats under standard conditions. I just can't understand that even you're agreeing that burning is exothermic than why is STILL statement number one correct? Even if it has nothing to do with the reaction at RT. It's wrong at first place. 
So do you mean that even if the first statement is literally wrong, we just have to answer the question in the sense in which it's being asked?

So do you mean that even if the first statement is literally wrong, we just have to answer the question in the sense in which it's being asked?

I did not agree that burning MUST be exothermic, though i might have implied that it is generally so. you are breaking very strong nitrogen triple bonds here, so you'd need very much energy to do so relative to the energy released when forming the new bonds. if you have a look at the standard enthalpy of formation of NO, which is equivalent to your rxn here(construct equations to satisfy yourself); you'd find that it is endothermic.
the point is again like what plato said, you'd need to think outside the box a little bit. yes, generally combustion is exothermic, but have you thought of why that must be? how can you change that?
and even if a reaction is exothermic, and spontaneously feasible, you still might not observe it at RT because of kinetic barrier. a good example is burning of wood. why isn't your wooden chair(presumably you have one) not burning now, eventhough like you said, combustion is exothermic(it is in this case).
(edited 13 years ago)
Original post by Zishi
Sorry for not mentioning about it before but it's a three-statement multiple completion item.
Here's the heading for this question:

Here's the heading for this question:

so in that case then, your answer is C (as statements 2 and 3 are correct). Though it's a poor question as it's only statement 2 that really answers the question. Statement 3 is true but irrelevant
Original post by Zishi
But isn't reaction with oxygen aka burning? 

Zishi, not all reaction with oxygen is burning (combustion).
Take for instance the corrosion of metals in air. This is a slow process of oxidation, which certainly cannot be described as burning.
The reaction of nitrogen with oxygen could also included as simple oxidation.
Combustion requires that the reaction with oxygen be self-sustaining. very exothermic and rapid.
Materials that undergo combustion almost invariably produce a vapour which combines exothermically with oxygen via a free radical process.
Original post by charco
Zishi, not all reaction with oxygen is burning (combustion).
Take for instance the corrosion of metals in air. This is a slow process of oxidation, which certainly cannot be described as burning.
The reaction of nitrogen with oxygen could also included as simple oxidation.
Combustion requires that the reaction with oxygen be self-sustaining. very exothermic and rapid.
Materials that undergo combustion almost invariably produce a vapour which combines exothermically with oxygen via a free radical process.
Take for instance the corrosion of metals in air. This is a slow process of oxidation, which certainly cannot be described as burning.
The reaction of nitrogen with oxygen could also included as simple oxidation.
Combustion requires that the reaction with oxygen be self-sustaining. very exothermic and rapid.
Materials that undergo combustion almost invariably produce a vapour which combines exothermically with oxygen via a free radical process.
Now Zishi, here is your genuine expert...
shengoc and I will go back to our sandpit

Original post by shengoc
I did not agree that burning MUST be exothermic, though i might have implied that it is generally so. you are breaking very strong nitrogen triple bonds here, so you'd need very much energy to do so relative to the energy released when forming the new bonds. if you have a look at the standard enthalpy of formation of NO, which is equivalent to your rxn here(construct equations to satisfy yourself); you'd find that it is endothermic.
the point is again like what plato said, you'd need to think outside the box a little bit. yes, generally combustion is exothermic, but have you thought of why that must be? how can you change that?
and even if a reaction is exothermic, and spontaneously feasible, you still might not observe it at RT because of kinetic barrier. a good example is burning of wood. why isn't your wooden chair(presumably you have one) not burning now, eventhough like you said, combustion is exothermic(it is in this case).
the point is again like what plato said, you'd need to think outside the box a little bit. yes, generally combustion is exothermic, but have you thought of why that must be? how can you change that?
and even if a reaction is exothermic, and spontaneously feasible, you still might not observe it at RT because of kinetic barrier. a good example is burning of wood. why isn't your wooden chair(presumably you have one) not burning now, eventhough like you said, combustion is exothermic(it is in this case).
Thank you. You applied Hess's law over here, right? As bond enthalpy of N-N triple bond is very large, that's why this reaction is endothermic?
Original post by Plato's Trousers
so in that case then, your answer is C (as statements 2 and 3 are correct). Though it's a poor question as it's only statement 2 that really answers the question. Statement 3 is true but irrelevant
The answer in mark scheme is A anyway. So according to mark scheme all options are correct.
Original post by charco
Zishi, not all reaction with oxygen is burning (combustion).
Take for instance the corrosion of metals in air. This is a slow process of oxidation, which certainly cannot be described as burning.
The reaction of nitrogen with oxygen could also included as simple oxidation.
Combustion requires that the reaction with oxygen be self-sustaining. very exothermic and rapid.
Materials that undergo combustion almost invariably produce a vapour which combines exothermically with oxygen via a free radical process.
Take for instance the corrosion of metals in air. This is a slow process of oxidation, which certainly cannot be described as burning.
The reaction of nitrogen with oxygen could also included as simple oxidation.
Combustion requires that the reaction with oxygen be self-sustaining. very exothermic and rapid.
Materials that undergo combustion almost invariably produce a vapour which combines exothermically with oxygen via a free radical process.
So does it means that I can't describe it as burning?

Original post by Plato's Trousers
Now Zishi, here is your genuine expert...
shengoc and I will go back to our sandpit
shengoc and I will go back to our sandpit

Cool, thanks anyway!

sorry, quite right, the answer is A
All your talk of burning (which is always exothermic) confused me. The reaction
is indeed endothermic
All your talk of burning (which is always exothermic) confused me. The reaction
is indeed endothermic
(edited 13 years ago)
Original post by Plato's Trousers
sorry, quite right, the answer is A
All your talk of burning (which is always exothermic) confused me. The reaction
is indeed endothermic
All your talk of burning (which is always exothermic) confused me. The reaction
is indeed endothermic
Sorry for asking this again and again, but just last time - this reaction IS NOT 'burning', right?
Original post by Zishi
Sorry for asking this again and again, but just last time - this reaction IS NOT 'burning', right?
correct.
Some reactions with oxygen are burning, yes. But many are not, charco gave the example of rusting. You wouldn't call that burning, would you?
Original post by Plato's Trousers
correct.
Some reactions with oxygen are burning, yes. But many are not, charco gave the example of rusting. You wouldn't call that burning, would you?
Some reactions with oxygen are burning, yes. But many are not, charco gave the example of rusting. You wouldn't call that burning, would you?
No, of course not!

Thanks to you and everyone for helping me with this!

Original post by Plato's Trousers
It's a multi-choice question ffs! Not all the answers are correct (that's the whole point!). You have to pick the right one!
Example:
What is a dog?
A a four legged mammal
B a glass measuring instrument
C a country in Asia
(clue : only one of those is correct)
Example:
What is a dog?
A a four legged mammal
B a glass measuring instrument
C a country in Asia
(clue : only one of those is correct)
Is it B? do we use it to measure out chemicals...
Quick Reply
Related discussions
- best revision resources for N5/H/AH
- How did you find the biology paper?
- I spent so much time revising and all for nothing - Computer science (Eduqas)
- National 5 Geography 2023
- University of Birmingham - The School of Engineering Mathematics Aptitude Test
- How can u help
- how to get better at maths
- NHS pre-interview assessment
- Edexcel AS Further Maths Options (19th May 2023)
- National 5 Chemistry
- core maths
- (Serious) A levels are too easy
- IGCSE Mandarin
- here we go againnn........
- Which a level is better option???
- Catered accommodation at Sheffield University?
- what do you think a 7 would be in this maths paper?
- Higher chemistry
- How to avoid stupid mistakes maths
- Edexcel maths
Latest
Last reply 8 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 9 minutes ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 12 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 13 minutes ago
Official: University of Leicester A199 (foundation year) 2024 entry applicantsPosted 14 minutes ago
University of Surrey academics back no confidence vote as 'morale is very low'Posted 17 minutes ago
Is SOAS the Right Choice for Comparative Literature MA? Insights Wanted!Last reply 20 minutes ago
Official University of the Arts London Applicant Thread for 2024Last reply 24 minutes ago
WJEC Eduqas English Literature prediction 2024Trending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products