OCR Salters Chemistry B F332 Advance Notice May 2011 Polymers on the move
Scroll to see replies
What is meant by Crystalline?
Crystalline
Regions have a high degree of order/ regular/ chains more aligned (1);
the chains move over each other less easily (1);
because there are stronger imf (1); (IGNORE “more imf”)
due to molecules being closer together (1)
Crystalline
Regions have a high degree of order/ regular/ chains more aligned (1);
the chains move over each other less easily (1);
because there are stronger imf (1); (IGNORE “more imf”)
due to molecules being closer together (1)
I don't think the idea of crystalline is in the spec anymore.
Original post by Bi0logical
What is meant by Crystalline?
Crystalline
Regions have a high degree of order/ regular/ chains more aligned (1);
the chains move over each other less easily (1);
because there are stronger imf (1); (IGNORE “more imf”)
due to molecules being closer together (1)
What is meant by Crystalline?
Crystalline
Regions have a high degree of order/ regular/ chains more aligned (1);
the chains move over each other less easily (1);
because there are stronger imf (1); (IGNORE “more imf”)
due to molecules being closer together (1)
Thanks!
But, a couple of weeks back I was doing an old spec past paper that had a question on crystalline polymers on it - I'd never heard the term crystalline, so I asked my teacher, and she said it was old spec and we didn't need to know it any more...
Can anyone confirm if it's still in the spec? I can't find it in the revision guider either!
Original post by Toshiya
Thanks!
But, a couple of weeks back I was doing an old spec past paper that had a question on crystalline polymers on it - I'd never heard the term crystalline, so I asked my teacher, and she said it was old spec and we didn't need to know it any more...
Can anyone confirm if it's still in the spec? I can't find it in the revision guider either!
But, a couple of weeks back I was doing an old spec past paper that had a question on crystalline polymers on it - I'd never heard the term crystalline, so I asked my teacher, and she said it was old spec and we didn't need to know it any more...
Can anyone confirm if it's still in the spec? I can't find it in the revision guider either!
Im pretty sure it isnt, it's not in any of the textbooks...
Original post by gozatron
I remember seeing crystalline and amorphous in the chemical ideas somewhere? Maybe not for F332..
I think it's been moved to A2.
Original post by Hudzy
Yeah I took it off the internet without realising it had an extra carbon. I posted an updated version but you probably missed it. I'll change the version on the old post to stop confusing people 
Here is the update anyway.

Here is the update anyway.
You are my hero! - For the revision polymer thing :L
Original post by gozatron
Anyone got a handy list of the reaction reagents and conditions we need to know?
The Jan 11 paper and Mark scheme would be pretty handy aswell
Thanks
The Jan 11 paper and Mark scheme would be pretty handy aswell

Thanks
Did you find a list of the reagents and conditions?
Original post by Toshiya
Anyone know exactly what points the mark scheme are looking for when they ask how ID-ID, PD-PD and H bonds are formed?
I always trip up on the wording with those questions!
I always trip up on the wording with those questions!
ID-ID
Electron movement causes an uneven distribution of charge. This induces a dipole in a neighbouring molecule, followed by attraction.
PD-PD
Simply one atom more electronegative than the other (explain that method if necessary) in a covalent bond, so PD-PD formed between different atoms of each molecule.
Hydrogen bond
Slightly positive H atom is attracted to the lone pair on an oxygen atom so H bond formed.
Key marking points underlined.
(edited 12 years ago)
Original post by icedragon
ID-ID
Electron movement causes an uneven distribution of charge. This induces a dipole in a neighbouring molecule, followed by attraction.
PD-PD
Simply one atom more electronegative than the other (explain that method if necessary) in a covalent bond, so PD (not PD-PD ) formed between molecules.
) formed between molecules.
Hydrogen bond
Slightly positive H atom is attracted to the lone pair on an oxygen atom so H bond formed.
Key marking points underlined.
Electron movement causes an uneven distribution of charge. This induces a dipole in a neighbouring molecule, followed by attraction.
PD-PD
Simply one atom more electronegative than the other (explain that method if necessary) in a covalent bond, so PD (not PD-PD
 ) formed between molecules.
) formed between molecules.Hydrogen bond
Slightly positive H atom is attracted to the lone pair on an oxygen atom so H bond formed.
Key marking points underlined.
Thanks! That's exactly what I wanted, whenever I answer a question like that I just waffle and still only manage to put in half of the points.

Original post by gozatron
Anyone got a handy list of the reaction reagents and conditions we need to know?
The Jan 11 paper and Mark scheme would be pretty handy aswell
Thanks
The Jan 11 paper and Mark scheme would be pretty handy aswell

Thanks
Try the spec, there's some, in that...
I bloody hate those reaction conditions.

EDIT: page 21.
(edited 12 years ago)
Original post by Toshiya
Thanks! That's exactly what I wanted, whenever I answer a question like that I just waffle and still only manage to put in half of the points. 

No problem. Just to note, it is PD-PD apparantly (I guess because its between 2 molecules and not intramolecular). Also remember to spell ID-ID and PD-PD out fully and correctly especially if there are any QWC marks.
Original post by icedragon
No problem. Just to note, it is PD-PD apparantly (I guess because its between 2 molecules and not intramolecular). Also remember to spell ID-ID and PD-PD out fully and correctly especially if there are any QWC marks.
Cheers.
And yeah, I know now to write them out fully... found out the hard way when I lost marks on a couple of mocks we did in class because I just put 'ID-ID'
Turns out if they ask for QWC marks on a question like that you often get them for spelling instantaneous and induced correctly.
Original post by Toshiya
Cheers.
And yeah, I know now to write them out fully... found out the hard way when I lost marks on a couple of mocks we did in class because I just put 'ID-ID'
Turns out if they ask for QWC marks on a question like that you often get them for spelling instantaneous and induced correctly.
And yeah, I know now to write them out fully... found out the hard way when I lost marks on a couple of mocks we did in class because I just put 'ID-ID'
Turns out if they ask for QWC marks on a question like that you often get them for spelling instantaneous and induced correctly.
Yep, or for linking your ideas together. So for example when describing how CO2 warms the Earth, you would get a QWC for saying "UV from the sun is absorbed by the Earth which heats the Earth and in turn it emits IR". Connection of ideas will get you that mark really.
Just wondering if anyone has any sort of notes on the entire specification, or sheets similar....
I could do with some cramming material to read through tomorrow
I could do with some cramming material to read through tomorrow

Worth knowing about free radical polymerisation (free radical addition):
The chain is initiated by radicals produced by the reaction between the alkene and an oxygen initiator. When these radicals meet a longer free radical is formed:
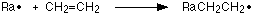
This chain continues:
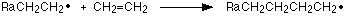
Until termination where two of the radicals meet making a final molecule:
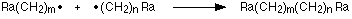
It's important to note that the chain termination is random, because 2 radicals could meet at any time (between long chains or short chains), so the polymer itself will be made up of chains of all different lengths.
The chain is initiated by radicals produced by the reaction between the alkene and an oxygen initiator. When these radicals meet a longer free radical is formed:
This chain continues:
Until termination where two of the radicals meet making a final molecule:
It's important to note that the chain termination is random, because 2 radicals could meet at any time (between long chains or short chains), so the polymer itself will be made up of chains of all different lengths.
(edited 12 years ago)
Original post by icedragon
Worth knowing about free radical polymerisation (free radical addition):
The chain is initiated by radicals produced by the reaction between the alkene and an oxygen initiator. When these radicals meet a longer free radical is formed:
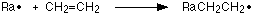
This chain continues:
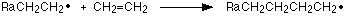
Until termination where two of the radicals meet making a final molecule:
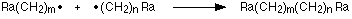
It's important to note that the chain termination is random, because 2 radicals could meet at any time (between long chains or short chains), so the polymer itself will be made up of chains of all different lengths.
The chain is initiated by radicals produced by the reaction between the alkene and an oxygen initiator. When these radicals meet a longer free radical is formed:
This chain continues:
Until termination where two of the radicals meet making a final molecule:
It's important to note that the chain termination is random, because 2 radicals could meet at any time (between long chains or short chains), so the polymer itself will be made up of chains of all different lengths.
icedragon you really are a respected and adored member, if it wasn't for you and hudsy i wouldn't have had a shot at this exam
THANKS

Original post by Toshiya
icedragon... I've never heard of free radical polymerisation.
I'll be having words with my chemistry teachers when we go back if that comes up tomorrow and I can't do it >_>
I'll be having words with my chemistry teachers when we go back if that comes up tomorrow and I can't do it >_>
It's basically the actual mechanism for addition polymerisation (it's how they add together). I think it's in the syllabus as it's in some of my guides but if it isn't, it could quite possibly be useful for the advanced notice question. I quote from the advanced notice:
"These are polymerised by a free radicalinitiated polyaddition." - from the Bonding polyalkenes section.
So they could very possibly ask you about the mechanism I explained.
Original post by bugsymalone
icedragon you really are a respected and adored member, if it wasn't for you and hudsy i wouldn't have had a shot at this exam
THANKS
THANKS

No problem
 . If there are any other mini-topics you would like explaining just shout. And what baffoon neg'd you?
. If there are any other mini-topics you would like explaining just shout. And what baffoon neg'd you? 
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- OCR Chemistry B (Salters) - Anybody doing this?
- Revision website for OCR B (salters) chemistry
- Is it possible to change sixth forms now (in year 12)?
- Analysis of Advanced Notice Article for Chemistry B Salters
- chemistry
- A Level Chemistry (OCR B Salters Chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- How to get A/A* in A level chemistry
- A Level Exam Discussions 2023
- A Level Chemistry Mark Scheme
- OCR B A-level Physics Paper 1 Advancing Physics (H557/01) - 24th May 2023 [Exam Chat]
- Politics or religious studies for A-level? Urgent!!
- AS and A level urgent help & advice
- OCR A GCSE Chemistry Paper 4 Higher Tier (J248 04) - 13th June 2023 [Exam Chat]
- How to increase grade in chemistry
- A Level OCR Salters Chemistry B
- GCSE Exam Discussions 2024
- OCR A-Level Chemistry B Paper 1 (H433/01) - 12th June 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 3 Higher Tier (J248 03) - 22nd May 2023 [Exam Chat]
Latest
Last reply 1 minute ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2839
Last reply 1 minute ago
Please Help! Seek input on University selection for FinanceLast reply 1 minute ago
Official University of Edinburgh Applicant Thread for 2024Last reply 4 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 5 minutes ago
Graded Unit Case Study HNC BusinessLast reply 7 minutes ago
Kingston or Westminster university for architecture?Last reply 11 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1256
Last reply 12 minutes ago
Official: University of Nottingham A100 2024 Entry Applicant threadLast reply 13 minutes ago
My Masters finishes in October and my PGCE stars in September will this be allowed?!Last reply 13 minutes ago
UWE Bristol - Discovering Higher and Degree Apprenticeships 2024Last reply 15 minutes ago
Official: University of Leicester A199 (foundation year) 2024 entry applicantsLast reply 17 minutes ago
Official University of St Andrews Applicant Thread for 2024Last reply 19 minutes ago
AQA A-level English Literature B Paper 1 (7717/1A-1B) - 24th May 2024 [Exam Chat]Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



