Equilibria, Energetics and Elements (F325) - June 2011 Exam.
Scroll to see replies
Guys lets make the Unofficial Mark Scheme Mk1
Please say if you have things to add, modify, or correct (theres lots!)
?)definition of lattice enthalpy: Energy given out when one mole of an ionic compound is formed from its constituent gaseous ions.[2]
?)Why is hydration enthalpy for Cl- exothermic? Bonds formed with deltaH+ on water molecules, heat given out.
?)Born Haber diagram of Enthalpy of solution, hydration, lattice enthalpy. 4 stages, incomplete answers: Mg2+(g) + 2Cl-(aq), Mg2+(aq) + 2Cl-(aq) [2]
?)
?)
graph question. Marks for calculating 3 half lives, ~200s, stating that constant half life means its 1st order. State that methanoic acid in excess, so [methanoic] is constant and can be disregarded in the rate equation. calculate k, answer and units= ?? s^-1
?)
?)
?)
?)
?)Magic tang question: use lactic acid because its pKa is close to our desired pH (within one unit), so it buffers better. I used henderson hasselbach equation, pKa = pH + log [A-]/[HA] or you can use the ka expression to work out [A-]/[HA], which (I got) ~0.49
I think it will be tangy because the buffering range (+/- 1 pH unit of pKa) certainly ecompasses the tangy pH of 3.55
?)
?)
?)
?)
?)
?)monomer unit for the complex ion polymer
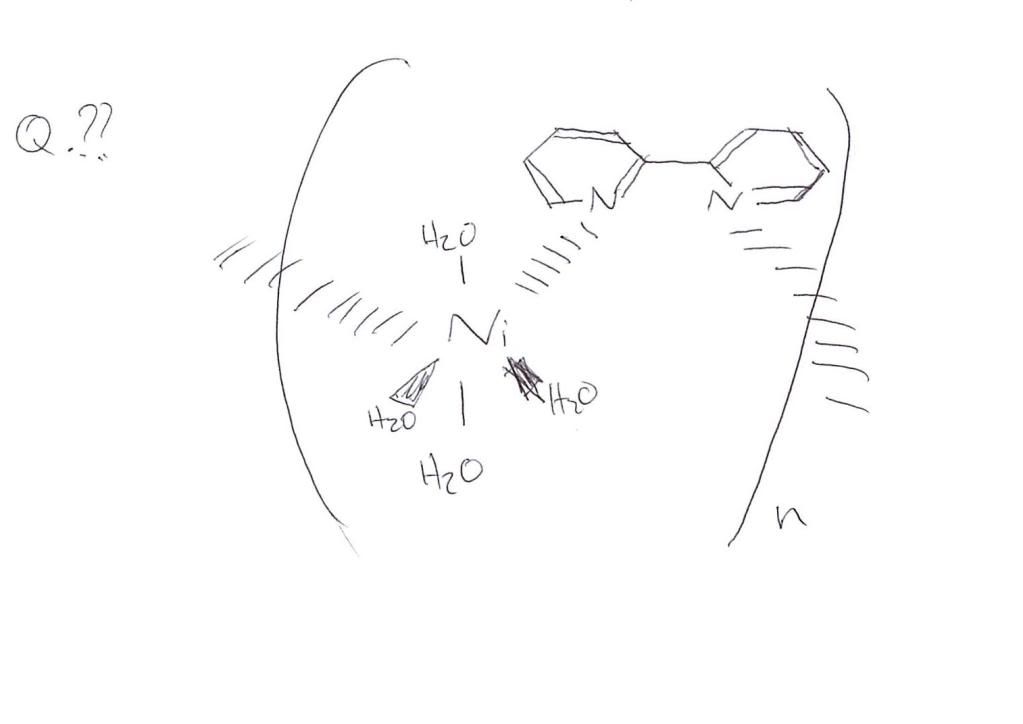
?)
?)
?)
?)
?)
?) % of Cu in Brass question
Equations with state symbols:
Cu (s) + 4HNO3 (l) --> Cu(NO3)2 (aq) + 2NO3- (aq) + 2NO + 2H2O
Cu2+ + 2I- (aq) --> CuI (s) + 0.5 I2 (aq)
(one more which i can't rememebr)
n(Cu) = 0.5n(I2) = n(S2O3)
titre of 24.98 x 10^-3 cm^3 or summin, 0.1moldm^-3, n (S2O3)=that, so n(Cu) = 24.98 x 10^-3 cm^3
so m(Cu) = nM = 24.98 x 10^-3 * 63.5 = 1.59 g of Cu in 2.8g or summin brass.
% by mass= 67 point something percent, it asked to 1dp.
Please say if you have things to add, modify, or correct (theres lots!)

?)definition of lattice enthalpy: Energy given out when one mole of an ionic compound is formed from its constituent gaseous ions.[2]
?)Why is hydration enthalpy for Cl- exothermic? Bonds formed with deltaH+ on water molecules, heat given out.
?)Born Haber diagram of Enthalpy of solution, hydration, lattice enthalpy. 4 stages, incomplete answers: Mg2+(g) + 2Cl-(aq), Mg2+(aq) + 2Cl-(aq) [2]
?)
?)
graph question. Marks for calculating 3 half lives, ~200s, stating that constant half life means its 1st order. State that methanoic acid in excess, so [methanoic] is constant and can be disregarded in the rate equation. calculate k, answer and units= ?? s^-1
?)
?)
?)
?)
?)Magic tang question: use lactic acid because its pKa is close to our desired pH (within one unit), so it buffers better. I used henderson hasselbach equation, pKa = pH + log [A-]/[HA] or you can use the ka expression to work out [A-]/[HA], which (I got) ~0.49
I think it will be tangy because the buffering range (+/- 1 pH unit of pKa) certainly ecompasses the tangy pH of 3.55
?)
?)
?)
?)
?)
?)monomer unit for the complex ion polymer

?)
?)
?)
?)
?)
?) % of Cu in Brass question
Equations with state symbols:
Cu (s) + 4HNO3 (l) --> Cu(NO3)2 (aq) + 2NO3- (aq) + 2NO + 2H2O
Cu2+ + 2I- (aq) --> CuI (s) + 0.5 I2 (aq)
(one more which i can't rememebr)
n(Cu) = 0.5n(I2) = n(S2O3)
titre of 24.98 x 10^-3 cm^3 or summin, 0.1moldm^-3, n (S2O3)=that, so n(Cu) = 24.98 x 10^-3 cm^3
so m(Cu) = nM = 24.98 x 10^-3 * 63.5 = 1.59 g of Cu in 2.8g or summin brass.
% by mass= 67 point something percent, it asked to 1dp.
(edited 12 years ago)
58/100 = A
plus a tangy sweet as compensation for mental torture.
plus a tangy sweet as compensation for mental torture.
Original post by dan_dan_dan
I'll be happy with half marks. Drew a wizard on the magic tang question, only relevant thing I could think of.
I left an note in the paper towards the examiner pleading with him/her to slip in a couple of marks my way. Not going to happen though.

Original post by pavan pong
is anybody going to put up a mark scheme or something
workin on it, help out!

Original post by pavan pong
it didnt say it was endothermic did it? it said it took place at zero so must it be exo as it requires no energy
Exactly what i thought
Original post by abloomfield
i got 33.8% hmm.. The ionic equation for stage 4 was just next
yes!!!!!!!!!!!!!!!!!!!!!!!!! Thats what i got
I thought the Br was a second order? :| !!!! Is that wrong!?!? It didnt have a constant half life did it?
Original post by Thuzz
sooo...
i failed that nicely
f324 retake next week better be merciful
i failed that nicely
f324 retake next week better be merciful
This & my physics re-sit is my last hope...
Original post by Rickesh
I thought the Br was a second order? :| !!!! Is that wrong!?!? It didnt have a constant half life did it?
It did I'm afraid, ~200s

Original post by susan23
Same ...actually believe it or not...i'm crying here just staring at the computer....93 is pretty low! You'll make it 

i thought i was the only one, cant believe an exam actually brought me to tears

Original post by Flint_09
yes!!!!!!!!!!!!!!!!!!!!!!!!! Thats what i got
I got that too !!
Original post by Student21
I left an note in the paper towards the examiner pleading with him/her to slip in a couple of marks my way. Not going to happen though.

PROPER LOL'ed now
From wikipedia: There are many different bronze alloys but modern bronze is typically 88% copper and 12% tin
Then I suppose my answer of 3.38% isn't correct.
Then I suppose my answer of 3.38% isn't correct.
Original post by Kalamari Dave
Guys lets make the Unofficial Mark Scheme Mk1
Please say if you have things to add, modify, or correct (theres lots!)
?)definition of lattice enthalpy: Energy given out when one mole of an ionic compound is formed from its constituent gaseous ions.[2]
?)Why is hydration enthalpy for Cl- exothermic? Bonds formed with deltaH+ on water molecules, heat given out.
?)Born Haber diagram of Enthalpy of solution, hydration, lattice enthalpy. 4 stages, incomplete answers: Mg2+(g) + 2Cl-(aq), Mg2+(aq) + 2Cl-(aq) [2]
?)
?)
graph question. Marks for calculating 3 half lives, ~200s, stating that constant half life means its 1st order. State that methanoic acid in excess, so [methanoic] is constant and can be disregarded in the rate equation. calculate k, answer and units= ?? s^-1
?)
?)
?)
?)
?)Magic tang question: use lactic acid because its pKa is close to our desired pH (within one unit), so it buffers better. I used henderson hasselbach equation, pKa = pH + log [A-]/[HA] or you can use the ka expression to work out [A-]/[HA], which (I got) ~0.49
I think it will be tangy because the buffering range (+/- 1 pH unit of pKa) certainly ecompasses the tangy pH of 3.55
?)
?)
?)
?)
?)
?)monomer unit for the complex ion polymer

?)
Please say if you have things to add, modify, or correct (theres lots!)

?)definition of lattice enthalpy: Energy given out when one mole of an ionic compound is formed from its constituent gaseous ions.[2]
?)Why is hydration enthalpy for Cl- exothermic? Bonds formed with deltaH+ on water molecules, heat given out.
?)Born Haber diagram of Enthalpy of solution, hydration, lattice enthalpy. 4 stages, incomplete answers: Mg2+(g) + 2Cl-(aq), Mg2+(aq) + 2Cl-(aq) [2]
?)
?)
graph question. Marks for calculating 3 half lives, ~200s, stating that constant half life means its 1st order. State that methanoic acid in excess, so [methanoic] is constant and can be disregarded in the rate equation. calculate k, answer and units= ?? s^-1
?)
?)
?)
?)
?)Magic tang question: use lactic acid because its pKa is close to our desired pH (within one unit), so it buffers better. I used henderson hasselbach equation, pKa = pH + log [A-]/[HA] or you can use the ka expression to work out [A-]/[HA], which (I got) ~0.49
I think it will be tangy because the buffering range (+/- 1 pH unit of pKa) certainly ecompasses the tangy pH of 3.55
?)
?)
?)
?)
?)
?)monomer unit for the complex ion polymer

?)
For the repeat unit I think you need:
Complex ion with 4 waters and the weird ligand thing
All inside square brackets with the charge
All inside curly brackets (like in the question) with an n for the repeak unit
the exam was really hard and on the last q if i read it propely could have got a few marks. i forgot to divide the moles for sodium thiosulfate by 2 as cu was only one mole
Original post by Kalamari Dave
It did I'm afraid, ~200s 

I didnt get that, nor did a few people I asked, they said its not a first order, as it didnt have a constant half life. when i worked it out i didnt get a constant half life?
Original post by Flint_09
yes!!!!!!!!!!!!!!!!!!!!!!!!! Thats what i got
so did I !!! but I heard in real life brass has approx 60% of cupper lol
Original post by Rickesh
I thought the Br was a second order? :| !!!! Is that wrong!?!? It didnt have a constant half life did it?
I got 2nd order aswell, because it didnt have a constant half life --the time went from around 190 to 400 which means it hasnt doubled = no constant half life?
I may be wrong though?
Well, I think I got about 55ish mark in this paper. Hopefully that'll equate to at least a high C. My mind just went blank and I swear a lot of the stuff I've never seen before. GG OCR.
Guys, if you write down a load of correct working and the correct answer but cross out the right answer and some of the working and put down a wrong answer instead, do you think you get to keep a few of those marks? I shot myself in the foot like that on the final question, so annoyed.. :/
Quick Reply
Related discussions
- Grade Growth Chronicles | From C's to A's (23-24)
- A-level Exam Discussions 2024
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- My attempt at not sabotaging my future career!
- GCSE Exam Discussions 2024
- Which exam boards offering biology Alevel do NOT have a practical exam?
- Day after masturbation
- Edexcel IGCSE Chemistry | PAPER 1
- Audio files for GCSE French Listening??
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- As level chemistry
- AQA Environmental science paper 2
- Energetics chem question
- Chemistry paper 2 igcse edexcel
- PSYB4 JUN11 mark scheme?
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- Canada vs UK
- chemistry as level equilibria help
- Hard friction question
- Edexcel Past Papers
Latest
Last reply 1 minute ago
Amazon Apprenticeships 2024Last reply 1 minute ago
Loughborough or Cardiff for architecture?Last reply 2 minutes ago
Grade 9 English GCSE Creative Writing 40 Mark ExampleLast reply 2 minutes ago
Accenture Degree Apprenticeship 2024Last reply 3 minutes ago
Medical doctor degree apprenticeship 2024Last reply 5 minutes ago
Temporary Withdrawl/Suspension of studies and Student Fianance.Last reply 7 minutes ago
MPhil Advanced Computer Science Cambridge - 2024 EntryLast reply 9 minutes ago
Standard Chartered Apprenticeships 2024Last reply 9 minutes ago
How important is IFST (Institute of Food Science & Technology) AccreditationLast reply 9 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 5 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]


