Equilibria, Energetics and Elements (F325) - June 2011 Exam.
Scroll to see replies
Original post by BackDoorEntry
Off the top of my head:
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
magic tang was just the way they dressed up a buffer solution problem, nothing beyond our scope.
the polymer and enthalpy questions were synoptic and application of knowledge, and 20% of the paper is supposed to be synoptic.
the KOH is application of knowledge, and it was only a 'suggest' question, meaning there will more than likely be more than 1 accepted answer. stop whining, we all sat the same paper. complaining won't do anything - every year they scale the raw marks depending on how many people got whatever mark so the top 20% get an A, or whatever.
Original post by BackDoorEntry
Off the top of my head:
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
I'd prefer a memory test but sadly its all about application.
+Magic tang was applying knowledge of buffers and the relation of pKa and pH to an unknown situation. The question didn't ask what is magic tang.
+Ice melts when you heat it, thus deltaH is endothermic?
+ That is an A* synoptic question designed for those people who want an A* to prove they know what they're doing.
Original post by CrazyDaisy123
You know the buffers question.
Did you have to use one of the acid in the question?
... i rambled on about ethanoic acid
And what was the neutralisation ans?
Did you have to use one of the acid in the question?
... i rambled on about ethanoic acid

And what was the neutralisation ans?
It doesn't matter which acid you picked. I chose ethanoic as well
which neutralisation question?
Original post by Sereni
Pretty sure I have an A... I ****ed up by not multiplying the Cl- hydration by two, getting 2.27% for the percentage of copper, and some of the other weird questions. I didn't drop more than 20 marks though...
feelsgoodman
Original post by arvin_infinity
Those people who think they have done well on this shocking paper (Hahahah u must be joking ! )
If u actually have done well..let us know how
Am really curious even if I'd known its gonna be this weird crappy paper I probably wouldnt have been able to prepare for it!! Did so many pastpapers but it was not any similar whatsoever
If u actually have done well..let us know how
Am really curious even if I'd known its gonna be this weird crappy paper I probably wouldnt have been able to prepare for it!! Did so many pastpapers but it was not any similar whatsoever
Think i dropped 8 marks on the last question, three on the electrode decomposing one and the ligand polymer question. Other than that i found it quite an easy paper
Original post by Sereni
Magic tang was 1 mark and it was just that acids are siur i think, koh i agree no clue, i managed to work out change in enthalpy with whatever they gave us (cant remember) so it cant have been impossible, and the polymer bidentate one was really just application of knowledge, you know it canform two coordinate bonds, you know the formula, you should be able to guess what its like, i think i screwed that one up coz of lack of time but i figured it out as i was leaving the exam
Magic tang was one mark? Wasnt it a large structured question? I had to keep on reading it to see if there was anything I missed!
None of it is impossible, but for an A level exam, some crucial bits were unecessary, and somewhat distant from the specification... which is just not on.
The polymer should have been drawn similar to this (but obviously with Nickel instead of Silver)
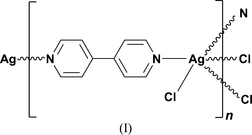

Does anyone know the equations for NiCd battery when recharging??
and the ionic equations at the negative and positive electrodes making hydrogen and oxygen...was it in a KOH fuel cell... or something??
and the ionic equations at the negative and positive electrodes making hydrogen and oxygen...was it in a KOH fuel cell... or something??
(edited 12 years ago)
Original post by BackDoorEntry
Off the top of my head:
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
+Magic tang.
+The KOH decomposing at the terminals.
+Determining change in enthalpy from.. well no values.
+Polymer bidentate complex ions??
I'd need to see the paper again to bring up all the ones I thought 'WTF IS OCR DOING??' questions.
magic tang - agreed!
KOH - agreed, ridiculous! even my chemistry teacher was confused by that...
enthalpy change - which one?
bidentate ligands - thought that wasn't a BAD question, since it's a straight extension of work that's in the textbook and on the syllabus, though the drawing of the polymer was NOT worth enough marks.
Original post by Hydro.ZX
Pretty sure I have an A... I ****ed up by not multiplying the Cl- hydration by two, getting 2.27% for the percentage of copper, and some of the other weird questions. I didn't drop more than 20 marks though...
feelsgoodman
feelsgoodman
Everyone has been getting like 67% on that question, and I got like 3% so if you got 2.27%, i feel a little better about that!
Original post by wiseman93
These are the people who I presume will regulate the examining bodies like OCR and make sure they don't blindside their candidates like this again right?
Well that's just dandy but I could really care less about future papers being fair and I'll be done with a-levels in a week or so so yeah not trying to sound selfish here but I think we should put our energy in complaining towards whoever will actually lower the grade boundaries accordingly.. OCR!!!
Original post by susan23
Dont bother complainign to OCR they wont change much...
complain to these guys [email protected] are the people who assess these exam baords
complain to these guys [email protected] are the people who assess these exam baords
These are the people who I presume will regulate the examining bodies like OCR and make sure they don't blindside their candidates like this again right?
Well that's just dandy but I could really care less about future papers being fair and I'll be done with a-levels in a week or so so yeah not trying to sound selfish here but I think we should put our energy in complaining towards whoever will actually lower the grade boundaries accordingly.. OCR!!!
Quoted from http://www.ofqual.gov.uk/
It is our duty to ensure all learners get the results they deserve and that their qualifications are correctly valued and understood, now and in the future.
(edited 12 years ago)
Original post by BA1
The polymer should have been drawn similar to this (but obviously with Nickel instead of Silver)


Er...but the square brackets would surely be outside the rest of it? but otherwise, agreed.
Original post by Sceaga
Original post by Sceaga
k=0.00342?? anyone else get something like this
I got same result for k
Original post by BA1
The polymer should have been drawn similar to this (but obviously with Nickel instead of Silver)


that looks like a ****ing alien hand
Original post by MoTheMedic
I'd prefer a memory test but sadly its all about application.
+Magic tang was applying knowledge of buffers and the relation of pKa and pH to an unknown situation. The question didn't ask what is magic tang.
+Ice melts when you heat it, thus deltaH is endothermic?
+ That is an A* synoptic question designed for those people who want an A* to prove they know what they're doing.
+Magic tang was applying knowledge of buffers and the relation of pKa and pH to an unknown situation. The question didn't ask what is magic tang.
+Ice melts when you heat it, thus deltaH is endothermic?
+ That is an A* synoptic question designed for those people who want an A* to prove they know what they're doing.
Okay I agree with the first point. I guess the way I saw it was a bit different because it simply took a lot out of me to actually find out what the question was asking of me!
Second point, didn't the question just say that it melts at 0 degrees, no mention of heating it. I put endothermic anyway.
For the third point.. How on earth do you prepare for a question like that though? I did some extra reading, i visited content from other specifications, I read deeper into topics we covered. Getting those A* questions right are just too hard to bother with, with hindsight!
(edited 12 years ago)
Original post by Teva
Does anyone know the equations for NiCd battery when recharging??
and the ionic equations at the negative and positive electrodes making hydrogen and oxygen...was it in a KOH fuel cell... or something??
and the ionic equations at the negative and positive electrodes making hydrogen and oxygen...was it in a KOH fuel cell... or something??
It's the opposite way around to the way that ought to be feasible, since you are putting energy in not getting it out. So essentially the equilibira just lie to the other side they did in the first part, the discharging question. I realise this AFTERWARDS..
Original post by BA1
The polymer should have been drawn similar to this (but obviously with Nickel instead of Silver)


i did this but split the molecule so one ring coming off as each ligand. basically the same thing but moved over to the right a bit if you know what i mean?
I'm desperate to see the paper! I want peace of mind 

Original post by BackDoorEntry
Magic tang was one mark? Wasnt it a large structured question? I had to keep on reading it to see if there was anything I missed!
None of it is impossible, but for an A level exam, some crucial bits were unecessary, and somewhat distant from the specification... which is just not on.
None of it is impossible, but for an A level exam, some crucial bits were unecessary, and somewhat distant from the specification... which is just not on.
Oh i assumed that ppl were refering to the last bit of it comment on the prediction that the magic tang is due to ph, the rest of the question was standard stuff about juggling around ka pka ph and h and ****. I guess my only big problem was time pressure, give me another ten minuted and i probably couldnt finished and gone back to figure out the polymer question
To the guy asking about rechargeing you just went backwards in the full equation, basically reversing the reaction which happens when the battery discharges
Quick Reply
Related discussions
- Grade Growth Chronicles | From C's to A's (23-24)
- A-level Exam Discussions 2024
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- My attempt at not sabotaging my future career!
- GCSE Exam Discussions 2024
- Which exam boards offering biology Alevel do NOT have a practical exam?
- Day after masturbation
- Edexcel IGCSE Chemistry | PAPER 1
- Audio files for GCSE French Listening??
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- As level chemistry
- AQA Environmental science paper 2
- Energetics chem question
- Chemistry paper 2 igcse edexcel
- PSYB4 JUN11 mark scheme?
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- Canada vs UK
- chemistry as level equilibria help
- Hard friction question
- Edexcel Past Papers
Latest
Last reply 2 minutes ago
MPhil Advanced Computer Science Cambridge - 2024 EntryLast reply 3 minutes ago
Official University of Bristol Offer Holders Thread for 2024 entryLast reply 3 minutes ago
Medical doctor degree apprenticeship 2024Last reply 5 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 6 minutes ago
Am I allowed a Calculator for university Computer Science exams?Last reply 12 minutes ago
Haven't heard back from LSEPosted 13 minutes ago
Can someone provide key examples for A level Politics USA for each topic please?Last reply 15 minutes ago
Children to no longer be prescribed puberty blockers, NHS England confirmsLast reply 17 minutes ago
Inlaks, Commonwealth, and Other Scholarships for Indian Students 2024: ThreadLast reply 18 minutes ago
Official UCL Offer Holders Thread for 2024 entryLast reply 19 minutes ago
LSE accommodation Q and ALast reply 21 minutes ago
2024 entry A100 / A101 Medicine fastest and slowest offer sendersMedicine
860
Last reply 22 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 22 minutes ago
Can I drop out of college once I have an unconditional uni offer?Last reply 22 minutes ago
What do I need - I've a UK passport but live in EUTrending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 6 days ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]


