Help with two exam question F321
Group 2 elements also react with dilute hydrochloric acid.
Describe and explain the trend in reactivity of the Group 2 elements with dilute hydrochloric acid as the group is descended.
Solid SiO2 melts at 2230 °C. Solid SiCl4 melts at –70 °C. Neither of the liquids formed conducts electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference in melting points in terms of bonding and structure.
Really confused...
any help would be appreciated
Thankss
Describe and explain the trend in reactivity of the Group 2 elements with dilute hydrochloric acid as the group is descended.
Solid SiO2 melts at 2230 °C. Solid SiCl4 melts at –70 °C. Neither of the liquids formed conducts electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference in melting points in terms of bonding and structure.
Really confused...
any help would be appreciated
Thankss

Scroll to see replies
I'm doing the same exam 
I would think of Silicon like Carbon.
Si02, like C02 has a double bond, but two lone pairs of electron and two bonding pairs, which are more repulsive making it non linear. So it is a non polar molecule because of the difference in electronegativety. As it is non polar, it there are dipole-dipole forces acting, which are stronger than the Van der Waals forces acting on SiCl4, a polar molecule. Does that make sense?
The other one seems to be a standard reactivity question
Trend in reactivityi increases because as you go down the group
-Increasing atomic radius and electron shielding means there is less attraction acting on the outer shell electrons
-These factors outweigh increasing nuclear charge.
-This increases the first ionisation energy, meaning less energy is needed to lose and electron
-Which makes it more reactive, and it is easier to lose two electrons in the reaction
What year is the paper? I'd look up the markscheme if I were you.

I would think of Silicon like Carbon.
Si02, like C02 has a double bond, but two lone pairs of electron and two bonding pairs, which are more repulsive making it non linear. So it is a non polar molecule because of the difference in electronegativety. As it is non polar, it there are dipole-dipole forces acting, which are stronger than the Van der Waals forces acting on SiCl4, a polar molecule. Does that make sense?
The other one seems to be a standard reactivity question
Trend in reactivityi increases because as you go down the group
-Increasing atomic radius and electron shielding means there is less attraction acting on the outer shell electrons
-These factors outweigh increasing nuclear charge.
-This increases the first ionisation energy, meaning less energy is needed to lose and electron
-Which makes it more reactive, and it is easier to lose two electrons in the reaction
What year is the paper? I'd look up the markscheme if I were you.
Ok, well think about what you've learnt. It's essentially quite simple.
What happens when a metal (group 2 element) reacts with acid? You get a salt, and you get hydrogen (for example Mg and HCl would form MgCl and H2).
Now you would basically describe why reactivity of Group 2 elements increases as you go down the group. You would talk about three things: atomic radius (this increases as you go down the group, reducing the nuclear attraction on the outer shell electrons), electron shielding/screening effect (which increases as you go down the group, reducing the net attraction from the nucleus to the outer shell electrons) and the nuclear charge, which increases as you go down, but the other 2 factors outweigh the nuclear charge increase (this is what's in every test and mark scheme, learn how to describe it, and it's easy marks mate). Then say that this means less energy is required to remove electrons and form 2+ ions as you go down the group.
You know it all, you just need to add those 3 factors and the marks are yours.
Then the next question.
Ok, now this is a little harder, but again, look to what you've been taught.
Draw the molecules out if it helps. Both molecules are covalent compounds, you can tell because the elements are all non-metal. However, they have very different melting points. Therefore you can work out that they have different intermolecular forces working on them. Both are simple covalent structures.
SiO2 has a very high melting point. The molecules in the lattice are polar because of the non-linear shape and difference in electronegativities. There would be an additional and stronger intermolecular force (permanent dipole-dipole) which requires more energy to overcome, hence the high melting point.
You can also guess that SiCl4 molecules are in simple covalent lattice structures, where the only intermolecular force acting on them is weak van der Waals' forces, hence less energy required to overcome the forces and change state.
Original post by farahh
Describe and explain the trend in reactivity of the Group 2 elements with dilute hydrochloric acid as the group is descended.
What happens when a metal (group 2 element) reacts with acid? You get a salt, and you get hydrogen (for example Mg and HCl would form MgCl and H2).
Now you would basically describe why reactivity of Group 2 elements increases as you go down the group. You would talk about three things: atomic radius (this increases as you go down the group, reducing the nuclear attraction on the outer shell electrons), electron shielding/screening effect (which increases as you go down the group, reducing the net attraction from the nucleus to the outer shell electrons) and the nuclear charge, which increases as you go down, but the other 2 factors outweigh the nuclear charge increase (this is what's in every test and mark scheme, learn how to describe it, and it's easy marks mate). Then say that this means less energy is required to remove electrons and form 2+ ions as you go down the group.
You know it all, you just need to add those 3 factors and the marks are yours.
Then the next question.
Original post by farahh
Solid SiO2 melts at 2230 °C. Solid SiCl4 melts at –70 °C. Neither of the liquids formed conducts electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference in melting points in terms of bonding and structure.
Solid SiO2 melts at 2230 °C. Solid SiCl4 melts at –70 °C. Neither of the liquids formed conducts electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference in melting points in terms of bonding and structure.
Ok, now this is a little harder, but again, look to what you've been taught.
Draw the molecules out if it helps. Both molecules are covalent compounds, you can tell because the elements are all non-metal. However, they have very different melting points. Therefore you can work out that they have different intermolecular forces working on them. Both are simple covalent structures.
SiO2 has a very high melting point. The molecules in the lattice are polar because of the non-linear shape and difference in electronegativities. There would be an additional and stronger intermolecular force (permanent dipole-dipole) which requires more energy to overcome, hence the high melting point.
You can also guess that SiCl4 molecules are in simple covalent lattice structures, where the only intermolecular force acting on them is weak van der Waals' forces, hence less energy required to overcome the forces and change state.
(edited 12 years ago)
Really sorry, but ignore that first explanation, I was getting confused with H20
SiO2 is a giant covalent structure, which accounts for its high boiling point. These strong colvalent bonds need to be broken to melt, and they require more energy as opposed to SiCl4's simple molecular structure.
I think that question was really unfair, I dont remember learning about Si02 on the syllabus.
SiO2 is a giant covalent structure, which accounts for its high boiling point. These strong colvalent bonds need to be broken to melt, and they require more energy as opposed to SiCl4's simple molecular structure.
I think that question was really unfair, I dont remember learning about Si02 on the syllabus.

Original post by allwordsaredust
Really sorry, but ignore that first explanation, I was getting confused with H20
SiO2 is a giant covalent structure, which accounts for its high boiling point. These strong colvalent bonds need to be broken to melt, and they require more energy as opposed to SiCl4's simple molecular structure.
I think that question was really unfair, I dont remember learning about Si02 on the syllabus.
SiO2 is a giant covalent structure, which accounts for its high boiling point. These strong colvalent bonds need to be broken to melt, and they require more energy as opposed to SiCl4's simple molecular structure.
I think that question was really unfair, I dont remember learning about Si02 on the syllabus.

no you were right, just wikied it, changed my answer lol!
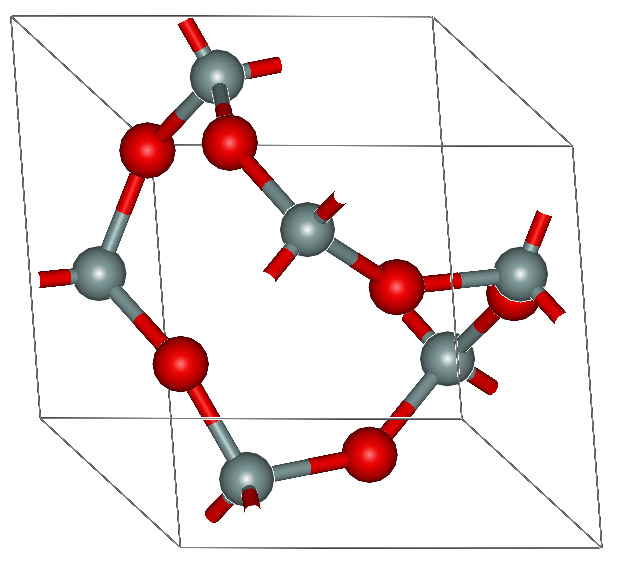
(edited 12 years ago)
Original post by farahh
Ok i get it now  Just need to try n remembering it
Just need to try n remembering it 
another question... how do you know its simple and not giant covalent structure.. is it something to do with the number of lone pairs?? :/
im soo going to fail chemistry.. Gulp.. :|
 Just need to try n remembering it
Just need to try n remembering it 
another question... how do you know its simple and not giant covalent structure.. is it something to do with the number of lone pairs?? :/
im soo going to fail chemistry.. Gulp.. :|
is it?
umm let me think.
The reason why both compounds had simple covalent lattices is because they aren't covalently bonded to each other, they had intermolecular forces attracting them to each other. SiO2 is non-linear, there's a large electronegativity difference, so it's polar, so attracted together by permanent dipole-dipole and vdW's, while SiCl4 is non-polar because of the symmetrical tetrahedral shape of the molecule, cancelling dipoles out. The only force between the molecules are vdW's.
Giant covalent lattices at AS will only ever be the carbon allotropes, so diamond, graphite and GCSE talks about buckminster fullerene. These are all just carbon atoms, but they don't exist by themselves like simple covalent, they are covalently bonded to each other. In diamond, the carbon atoms are bonded together in a tetrahedral shape (each carbon covalently bonded to 4 other carbons) in graphite the molecules are in layers, C atoms are bonded to 3 other carbons, and there is one free electron.
You shouldn't really get any questions going past those 2 giant covalent structures...
Original post by Phil90974
Solid SiO2 melts at 2230 °C. Solid SiCl4 melts at –70 °C. Neither of the liquids formed conducts
electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference
in melting points in terms of bonding and structure.
In your answer you should use appropriate technical terms, spelled correctly.
SiO2 is giant covalent (lattice)
SiCl4 is simple molecular (lattice)
van der Waals’ forces in SiCl4
Covalent bonds broken in SiO2
Forces OR bonds are stronger in SiO2 (than in SiCl4)
OR more energy is needed to break forces OR bonds in SiO2
(than in SiCl4)
electricity.
Suggest the type of lattice structure in solid SiO2 and in solid SiCl4 and explain the difference
in melting points in terms of bonding and structure.
In your answer you should use appropriate technical terms, spelled correctly.
SiO2 is giant covalent (lattice)
SiCl4 is simple molecular (lattice)
van der Waals’ forces in SiCl4
Covalent bonds broken in SiO2
Forces OR bonds are stronger in SiO2 (than in SiCl4)
OR more energy is needed to break forces OR bonds in SiO2
(than in SiCl4)
oh really? That's strange. OK, that's not in the textbook, I posted an image from wiki, I must have misunderstood it... That's very hard for an AS paper...
well thanks for the help.
Original post by Pride
oh really? That's strange. OK, that's not in the textbook, I posted an image from wiki, I must have misunderstood it... That's very hard for an AS paper...
well thanks for the help.
well thanks for the help.
Questions like this certainly arent rare? and this is all covered in the textbook. Have you done every past paper?
Original post by Phil90974
Questions like this certainly arent rare? and this is all covered in the textbook. Have you done every past paper?
well, both simple molecular and giant covalent are in the textbook.
However, it doesn't say how you know which one is which. Only 2 examples of giant covalent lattices are given in the textbook, diamond and graphite, which are both carbon allotropes. I'd have no way of knowing that SiO2 molecules are covalently bonded to each other in a lattice. The only thing that would suggest that to me is the very high melting point.
Original post by Pride
well, both simple molecular and giant covalent are in the textbook.
However, it doesn't say how you know which one is which. Only 2 examples of giant covalent lattices are given in the textbook, diamond and graphite, which are both carbon allotropes. I'd have no way of knowing that SiO2 molecules are covalently bonded to each other in a lattice. The only thing that would suggest that to me is the very high melting point.
However, it doesn't say how you know which one is which. Only 2 examples of giant covalent lattices are given in the textbook, diamond and graphite, which are both carbon allotropes. I'd have no way of knowing that SiO2 molecules are covalently bonded to each other in a lattice. The only thing that would suggest that to me is the very high melting point.
Main bits of info in the question: Neither of the liquids formed conducts
electricity. Suggest the lattice structure
The 4 main types covered in the book are, giant ionic, metallic, giant covalent, and simple molecular.
The fact that it does not conduct when liquid rules out giant ionic lattice, and metallic. This leaves giant covalent and simple molecular, and you should be aware that simple molecular have low boiling and melting points due to weak vdW and giant covalent have high melting and boiling points.
Do you have the CGP revision guide?
Original post by farahh
Thank you soo much Everyone 
This actually helped me alot :

This actually helped me alot :

Page 37 of the CGP revision guide is rather helpful with the table
Hey I have a question from past paper 2009 to do with which molecule could exist as a giant covalent structure. I narrowed it down to SiO2 and SiCl4 because I knew that Silicon was macromolecular. I concluded that it must be SiO2 because I could not see how SiCl4 could continue to make more covalent bonds so that it could have a giant covalent structure. I assumed that SiO2 CAN be giant covalent because the lone pair of electrons on oxygen can bond again with another Silicon atom. I wanted to know if my assumption is correct? (I did get it right btw but it was by process of elimination). Where as SiCl4 has no more electrons which is can bond with (covalently).
http://www.admissionstests.cambridgeassessment.org.uk/adt/digitalAssets/119145_BMAT_Sec_2_2009.pdf
Once again thanks
EDIT: wrong thread sorry
http://www.admissionstests.cambridgeassessment.org.uk/adt/digitalAssets/119145_BMAT_Sec_2_2009.pdf
Once again thanks

EDIT: wrong thread sorry

(edited 11 years ago)
How do you diffenterate from simple molecular and covalent
This was posted from The Student Room's iPhone/iPad App
This was posted from The Student Room's iPhone/iPad App
Original post by Jimmy20002012
How do you diffenterate from simple molecular and covalent
This was posted from The Student Room's iPhone/iPad App
This was posted from The Student Room's iPhone/iPad App
Simple molecular is covalent. So like CO2 is simple molecule the molecule floats around as simple molecules of CO2 gas. Whereas diamond or graphite or silicon dioxide is giant macromolecular. Still covalent bonding but each atom is bonded to another in a giant structure. Whereas simple molecular the only forces of interaction are intermolecular such as Van der walls.
Original post by Pride
well, both simple molecular and giant covalent are in the textbook.
However, it doesn't say how you know which one is which. Only 2 examples of giant covalent lattices are given in the textbook, diamond and graphite, which are both carbon allotropes. I'd have no way of knowing that SiO2 molecules are covalently bonded to each other in a lattice. The only thing that would suggest that to me is the very high melting point.
However, it doesn't say how you know which one is which. Only 2 examples of giant covalent lattices are given in the textbook, diamond and graphite, which are both carbon allotropes. I'd have no way of knowing that SiO2 molecules are covalently bonded to each other in a lattice. The only thing that would suggest that to me is the very high melting point.
I didn't know that SiO2 is a giant covalent compound, but it is in the textbook.
I was reading the textbook today, and found that on page 51 at the bottom of left column, "Instead the covalent bonds extend throughout the crystal in a giant lattice structure. An example of this is silicon dioxide, which forms quartz crystals. Silicon dioxide has a giant covalent structure."
(edited 11 years ago)
Original post by blue11_l
I didn't know that SiO2 is a giant covalent compound, but it is in the textbook.
I was reading the textbook today, and found that on page 51 at the bottom of left column, "Instead the covalent bonds extend throughout the crystal in a giant lattice structure. An example of this is silicon dioxide, which forms quartz crystals. Silicon dioxide has a giant covalent structure."
I was reading the textbook today, and found that on page 51 at the bottom of left column, "Instead the covalent bonds extend throughout the crystal in a giant lattice structure. An example of this is silicon dioxide, which forms quartz crystals. Silicon dioxide has a giant covalent structure."
oh okay it's in there, brilliant. I'm glad you're reading the book more carefully than I was.
 I study A2 now so I don't have it anymore.
I study A2 now so I don't have it anymore.Original post by allwordsaredust
I'm doing the same exam 
I would think of Silicon like Carbon.
Si02, like C02 has a double bond, but two lone pairs of electron and two bonding pairs, which are more repulsive making it non linear. So it is a non polar molecule because of the difference in electronegativety. As it is non polar, it there are dipole-dipole forces acting, which are stronger than the Van der Waals forces acting on SiCl4, a polar molecule. Does that make sense?
The other one seems to be a standard reactivity question
Trend in reactivityi increases because as you go down the group
-Increasing atomic radius and electron shielding means there is less attraction acting on the outer shell electrons
-These factors outweigh increasing nuclear charge.
-This increases the first ionisation energy, meaning less energy is needed to lose and electron
-Which makes it more reactive, and it is easier to lose two electrons in the reaction
What year is the paper? I'd look up the markscheme if I were you.

I would think of Silicon like Carbon.
Si02, like C02 has a double bond, but two lone pairs of electron and two bonding pairs, which are more repulsive making it non linear. So it is a non polar molecule because of the difference in electronegativety. As it is non polar, it there are dipole-dipole forces acting, which are stronger than the Van der Waals forces acting on SiCl4, a polar molecule. Does that make sense?
The other one seems to be a standard reactivity question
Trend in reactivityi increases because as you go down the group
-Increasing atomic radius and electron shielding means there is less attraction acting on the outer shell electrons
-These factors outweigh increasing nuclear charge.
-This increases the first ionisation energy, meaning less energy is needed to lose and electron
-Which makes it more reactive, and it is easier to lose two electrons in the reaction
What year is the paper? I'd look up the markscheme if I were you.
the mark schem says the sio2 is a covalent lattice whereas sicl4 is a simple molecule how is that possible?
Quick Reply
Related discussions
- GCSE Exam Discussions 2024
- A guide on how to get a 9 in GCSE Edexcel Business Studies
- LIBF Level 3 Certificate in Financial Studies
- Edexcel GCSE Mathematics Paper 3 (1MA1 3) - 13th November 2023 [Exam Chat]
- Getting ahead in Year 10!
- 1000+ A2-Level Biology Exam Questions
- GCSE options
- a level speaking advice
- i suck at a-level economics
- Politics a level person edexcel
- BA broadcast journalism
- gcse help
- Edexcel A Level German Paper 1: 9GN0 01 - 9 Jun 2022 [Exam Chat]
- Dental Nurse Zoom OSCE
- Biology AQA A Level
- should i start doing past papers or is it too early
- I'm an undergraduate law student at the University of Kent - Ask me anything!
- AQA A-level Drama and Theatre Studies (7262/W) - 7th June 2023 [Exam Chat]
- year 10 mocks
- A-Level English literature essays
Latest
Last reply 1 minute ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 1 minute ago
lloyds bank 2024 apprenticeshipLast reply 4 minutes ago
Official University College London Applicant Thread for 2024Last reply 7 minutes ago
Is it really such a bad thing to have an undefined relationshipLast reply 7 minutes ago
Official University of Bath Offer Holders Thread for 2024 entryLast reply 7 minutes ago
Official University of Edinburgh Applicant Thread for 2024Posted 9 minutes ago
London student accomodation - Studio available over the summerLast reply 10 minutes ago
National Probation ServiceLast reply 10 minutes ago
Anyone else get an offer for a Rolls Royce degree apprenticeship in Derby (2024)?Last reply 11 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadPosted 14 minutes ago
Lloyds of London Work Experience Year 12 2024Last reply 16 minutes ago
Best university to study Film studies/production?Last reply 18 minutes ago
Official UCL Offer Holders Thread for 2024 entryTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products


