Difference between sterioisomers and structural isomers!?
Sterioisomer : same structural formula but different 3d arrangement of atoms in space
structural isomer : same molecular forumla but different arrangement of atoms
To me they are identical ! obviously they are not
Please someone explain how they are different!

+rep
Sterioisomer : same structural formula but different 3d arrangement of atoms in space
structural isomer : same molecular forumla but different arrangement of atoms
To me they are identical ! obviously they are not
Please someone explain how they are different!

+rep
This sort of explains it well.

Here's two websites that explain the two things well. Let me know if you don't understand it!
Sterioisomer
http://www.chemguide.co.uk/basicorg/isomerism/geometric.html
Structural isomerism
http://www.chemguide.co.uk/basicorg/isomerism/structural.html
Sterioisomer : same structural formula but different 3d arrangement of atoms in space
structural isomer : same molecular forumla but different arrangement of atoms
To me they are identical ! obviously they are not
Please someone explain how they are different!

+rep
Structural isomers: same molecular formula, but different structural formulas. You have 3 types of structural isomer; chain, positional and function group.
Structural isomerism deals with the different arrangement of compounds, whereas, stereoisomerism has the same arrangement but a different spacial orientation within the arrangement. That is the difference.
There are 2 types of stereoisomerism; geometric and optical.
Edit: If you need further explanation, then please let me know.

Like it
 well answered
well answered You know the difference between structural and molecular formula?
Here's two websites that explain the two things well. Let me know if you don't understand it!
There are 2 types of stereoisomerism; geometric and optical.
Edit: If you need further explanation, then please let me know.
You have reached the limit of how many posts you can rate today!!!! OH well, will +rep you later

So lets look at this example:
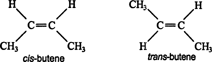
They both have the same molecular formula and same arrangement of atoms and therefore same structural formula
But they have different arrangement of atoms in space
So basically structural is in 2D but sterioisomer is in 3D
 well answered
well answered You have reached the limit of how many posts you can rate today!!!! OH well, will +rep you later

So lets look at this example:
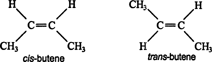
They both have the same molecular formula and same arrangement of atoms and therefore same structural formula
But they have different arrangement of atoms in space
So basically structural is in 2D but sterioisomer is in 3D
Yeh, you could say that, since Optical isomers (a type of stereoisomer) are non-superimposable and are mirror images of each other.

Stereoisomers are isomers with the same structural formula but with a different arrangement of atoms in space.
E/Z isomerism is an example of steroisomerism in the terms of restricted rotation about a double bond. With the requirement for two different groups to be attached to each Carbon of the C=C group.
Cis/Trans isomerism is a special case of E/Z isomerism where two substituent groups are the same.
Whenever there is E/Z isomerism there is Cis/ Trans they are basically the same thing but E/Z is a more modernly used term.
You seem to have the understanding of the concept, and so it really is thinking of it in a way that works best for you. As far as I can see, there is nothing wrong with thinking about it in that way.
Cis/Trans isomerism is a special case of E/Z isomerism where two substituent groups are the same.
Whenever there is E/Z isomerism there is Cis/ Trans they are basically the same thing but E/Z is a more modernly used term.
It isn't a difficult concept to understand, but it is highly unlikely that anything beyond the cis-trans system will be required for A-level. This is why cis-trans are what are generally taught at A-level.
On the OCR F322 specification you mostly need to know E/Z isomerism. Also show examples of Cis/ Trans isomerism. As i said they are the same thing except E/Z is more on the newer specifications.
Cis/Trans isomerism is a special case of E/Z isomerism where two substituent groups are the same.
Whenever there is E/Z isomerism there is Cis/ Trans they are basically the same thing but E/Z is a more modernly used term.
I was told that E/Z is the terminology for IUPAC, and Cis/Trans was just old, i.e. they're interchangeable.


I assume so yes. It's in all my OCR Revision guides and i think it's on the specification sheet. Cis/ Trans is old terminology yes. But E/Z is more of what they are getting towards now i think.
Its because compounds like BromoFluoroChloroEthene can't be named with cis/trans
Instead it is named with E/Z
Such as:
Cis but-2-ene is also pronounces E but-2-ene
E/Z isomerism is an example of steroisomerism in the terms of restricted rotation about a double bond. With the requirement for two different groups to be attached to each Carbon of the C=C group.
E/Z Can have different groups. Cis/trans need 2 substituent groups the same.
Such as:
Cis but-2-ene is now E but-2-ene.
Yeah, I get that - I thought you said that there's a difference in the chemistry rather than "Chemistry PCness"
 haha read up
haha read up 
ahh - didn't know that


Yeh not interchangeable I guess..Basically E/z introduced cuz they couldnt name complicated isomers when all group attached to carbons are different!
So what they do is they look for priorities and then change the complicated version of isomer to a simple version like cis/trans (e.g. higher priorities on different side is E) its beyond A-level to actually identify those priorities
Its because compounds like BromoFluoroChloroEthene can't be named with cis/trans
Instead it is named with E/Z
Such as:
Cis but-2-ene is also pronounces E but-2-ene
E/Z isomerism is an example of steroisomerism in the terms of restricted rotation about a double bond. With the requirement for two different groups to be attached to each Carbon of the C=C group.
E/Z Can have different groups. Cis/trans need 2 substituent groups the same.
I know all of this, but didn't realise that you had word perfectly quoted your specification in your original post in this thread. Therefore, I can only apologise for what may have mislead people.
However, in my specification, it is only required to have an appreciation of the E/Z system but not required to be able to apply anything beyond the cis-trans system as molecules of such complexity won't come up in the exam.
So what they do is they look for priorities and then change the complicated version of isomer to a simple version like cis/trans (e.g. higher priorities on different side is E) its beyond A-level to actually identify those priorities
Not beyond A level. It's in the AQA spec...
3.2.9: Students should:
•
know that the alkenes can exhibit E-Z stereoisomerism
•
be able to draw the structures of E and Z isomers
•
understand that E-Z isomers exist due to restricted rotation about the C=C bond
3.4.4: Students should:
•
know and understand the meaning of the term structural isomerism
•
know that E-Z isomerism and optical isomerism are forms of stereoisomerism
3.2.9: Students should:
•
know that the alkenes can exhibit E-Z stereoisomerism
•
be able to draw the structures of E and Z isomers
•
understand that E-Z isomers exist due to restricted rotation about the C=C bond
3.4.4: Students should:
•
know and understand the meaning of the term structural isomerism
•
know that E-Z isomerism and optical isomerism are forms of stereoisomerism
My bad I meant to say its not in the OCR spec!
Dont get me wrong here, e/z is in the OCR spec but identifying them NOT in the syllabus
or if you had two molecules both as CHCl-CHCl one could have both Cl on the same side ie: Cl Cl Cl H
C=C OR C=C
H H H Cl
as a result of the double bond the molecules cannot rotate (like Alkanes) so despite having the same structural formula they have a different arrangement of atoms in space therefore it is a stereoisomer
or if you had two molecules both as CHCl-CHCl one could have both Cl on the same side ie: Cl Cl Cl H
C=C OR C=C
H H H Cl
as a result of the double bond the molecules cannot rotate (like Alkanes) so despite having the same structural formula they have a different arrangement of atoms in space therefore it is a stereoisomer
This thread is 5 years old.
Quick Reply
Related discussions
- E/Z stereoisomerism with alcohols
- Why is 1,1,2-trimethylpropane not an isomer of hexane
- Reaction Products
- chemistry question!
- Organic chemistry question
- What type of isomerism do Ketones and Functional groups have?
- A level Chemistry help
- A level Chemistry OCR HELP
- GCSE Chemistry Past Paper Question
- Unit 14: Applications of Organic Chemistry
- isomerism a level chemistry urgent help
- Why is1-methylpentane not an isomer of Hexane
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio)
- AQA Chemistry Alevel Organic synthesis
- Organics AS MCQ
- Isomerism
- iupac naming of chromanones
- Mass Spectrometry question
- AQA Chem Unit 1 May 22nd 2015 *OFFICIAL THREAD*
- Help urgent spectroscopy
Latest
Last reply 1 minute ago
amazon apprenticeship Buying and MerchandisingLast reply 3 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 12 minutes ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 20 minutes ago
Based on 2023 GCSEs, will grade boundaries be higher or lower in 2024?13
Last reply 21 minutes ago
Apprenticeship needs a 4 in scienceLast reply 23 minutes ago
Should I accept my offer at LCF or wait another year and apply for CSM again?Last reply 25 minutes ago
GCSE geography case studies CIE -urgent help neededLast reply 27 minutes ago
Is it really such a bad thing to have an undefined relationshipLast reply 31 minutes ago
Best SEO Service Company In USATrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these products



