AQA AS Chemistry, unit 1
Scroll to see replies
Remember with the ideal gas equation..
Pressure must be in Pa
Volume must be in m3
Constant = 8.31KJmol-1
Temp must be in K (to convert from degrees add 273)
Posted from TSR Mobile
Pressure must be in Pa
Volume must be in m3
Constant = 8.31KJmol-1
Temp must be in K (to convert from degrees add 273)
Posted from TSR Mobile
Original post by a.jiwa
concentration is moldm^3
and volume is dm^3 (you divide by 1000 because they usually give it in cm^3)
remember that or they might trick you!
and volume is dm^3 (you divide by 1000 because they usually give it in cm^3)
remember that or they might trick you!
Oh yeah thank you so much, I would have completely forgotten that

Quick question. When giving electronic configurations of atoms can you put in the Noble gas in brackets and then the rest of the configuration?
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
Does anyone know how to convert units in the PV=nRT equation. I know degrees to k is +273. and if pressure is kPa to multiply by 1000. but the volume confuses me between the dm^3, m^3 and cm^3 

Original post by IWantSomeMushu
Quick question. When giving electronic configurations of atoms can you put in the Noble gas in brackets and then the rest of the configuration?
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
I got told no. Just to put the whole electron configuration to be sure.
Original post by IWantSomeMushu
Quick question. When giving electronic configurations of atoms can you put in the Noble gas in brackets and then the rest of the configuration?
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
E.g. for Rubidium - [Kr] 5s1
instead of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
I was told if it asks for the full electron configuration don't put the noble gas in

Original post by swinnie0
It's to do with it being more stable with a full/half full 3d orbital because it's a transition metal. It's in chem5, i wasn't aware you needed to know it in chem 1 

I highly doubt it will come up tomorrow but i read it in the text book, didnt understand it at all but memorized it just incase! I havent seen it and others have informed me that they havent seen it in spec or past papers.
As long as aqa dont decide to mess with us then i dont think it will be in the paper. but better safe than sorry and it doesnt hurt to learn a sentence does it?


Original post by Amy Corthine
Does anyone know how to convert units in the PV=nRT equation. I know degrees to k is +273. and if pressure is kPa to multiply by 1000. but the volume confuses me between the dm^3, m^3 and cm^3 

cm3 /1000 -> dm3 /1000 -> m3

Posted from TSR Mobile
Original post by swinnie0
I was told if it asks for the full electron configuration don't put the noble gas in 

Ugh, more work for just one mark.
Original post by Areen5
Oh yeah thank you so much, I would have completely forgotten that 

your welcome! goodluck tomorrow

Original post by homefind
Yes.
Centimetres
Divided by 1000
Decimetres
Divided by 1000
Metres
Original post by Paulineuh
I used to have this coloured periodic table which showed which is non-metal and metal xD That's how I just remembered it 
Posted from TSR Mobile

Posted from TSR Mobile
Original post by homefind
Because it isn't a metal?lol I don't know how to explain it lol, anyone else? erm well it isn't a transition and isn't in group 1 or 2
Posted from TSR Mobile
Posted from TSR Mobile
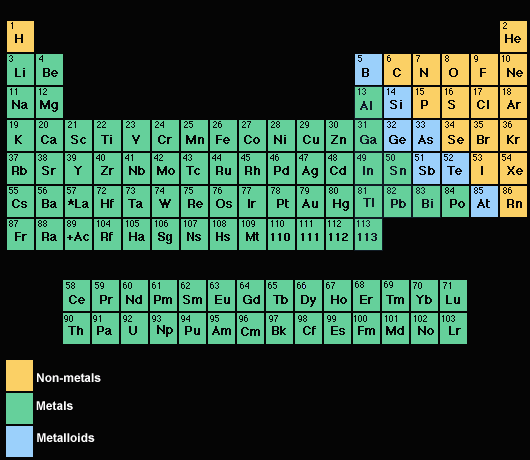
This does it pretty well.
I'm gonna remember the staircase with the image of a running pencil.
Pencil (carbon) to Running (Running / Rn / Radon)
Meh, my brain works differently.
Original post by NabRoh
This does it pretty well.
I'm gonna remember the staircase with the image of a running pencil.
Pencil (carbon) to Running (Running / Rn / Radon)
Meh, my brain works differently.

This does it pretty well.
I'm gonna remember the staircase with the image of a running pencil.
Pencil (carbon) to Running (Running / Rn / Radon)
Meh, my brain works differently.
Awesome

Posted from TSR Mobile
Do atomic model questions ever come up?
Posted from TSR Mobile
Posted from TSR Mobile
Original post by homefind
Not that I know of/seen on an actual paper. However, at my school we did a mini-end of unit test once and there was a question on atomic model.

Original post by homefind
Sorry what's that?

Original post by Paulineuh
Not that I know of/seen on an actual paper. However, at my school we did a mini-end of unit test once and there was a question on atomic model. 

What was the Q like?
Posted from TSR Mobile
Original post by maryamnc
Sorry what's that? 

The spec states you need to be aware about atomic models (like Plum Pudding etc...) but I haven't seen Qs asking about them before.
Posted from TSR Mobile
Original post by homefind
No.
Hey does anyone know any good sites for revising organic chemistry.
fractional distillation and cracking and all that kind of stuff?
fractional distillation and cracking and all that kind of stuff?
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Oxford AQA International A-Level past papers
- GCSE Exam Discussions 2023
- oxfordAQA chemistry papers
- AS/A Level Chemistry Study Group 2023/2024
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- chemistry as percentage uncertainty question
- chemistry aqa a-level question help
- tips please!
- how to revise ?
- Chemistry a level enthalpy
- a level study : BLOG
- btec applied science extended certificate aqa
- Life and Health science (step up science equivalent)
- I'm confused about my As levels
- GCSE 2023 Grade Boundaries (All Exam Boards)
- How do you get A's in Biology A levels
- How many GCSEs are you getting?
Latest
Last reply 3 minutes ago
LSE undergraduate accommodationLast reply 9 minutes ago
The Official Cambridge Applicants for 2025 Entry ThreadLast reply 9 minutes ago
Airbus degree apprenticeships 2024Last reply 10 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 27 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawTrending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



