This discussion is now closed.
Check out other Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- IAL repeats cash in.
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- Self-teaching Chemistry A-level (as a private candidate)?
- Official thread - May/June 2023 Edexcel IAL
- Switching from A level to IAL
- A-level Biology Study Group 2022-2023
- Trek To The End Of Year 13 ❀◕ ‿ ◕❀
- Uniq 2023
- School is killing me - Y11 "GYG" 2022
- BTEC Results FINAL Certificate
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- Best timetable for studying 4 subjects?
- Edexcel A-Level French Paper 2 (9FR0 02) - 19th June 2024 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- A-level Chemistry Study Group 2022-2023
- iria's 'grow your grades' blog :)
Edexcel Chemistry A2 Unit 5 ~ Wednesday 19th June 2013 (Now Closed)
Scroll to see replies
Original post by Knoyle quiah
Can someone explain how to work out the answer for this? answer is D but how, i thought sodium carbonate gets rid of acid impurities, and i havnt come across sodium sulphate as a drying agent
The sodium carbonate will clearly remove SO2 and HBr since they're acids.
Then it hints that sodium sulphate is a drying agent by telling you that it is anhydrous and so it's not going to affect anything but the water.
This leaves you making an educated guess that Bromine would be removed by the sodium carbonate (like this
 )so the answer must be D.
)so the answer must be D.As far as I know we don't have to actually learn or remember that sodium sulphate is a drying agent or that sodium carbonate removes bromine, you just have to use the hints they give you to try and work it out.
(edited 10 years ago)
I don't know if too little sleep is taking its toll but I don't understand the answer to this question. Why is that Carbon chiral? I thought it needed four different groups bonded to it but that only has 3 groups (2 of which are the same as far as I can tell).
Does anyone know?
Thank you.

Does anyone know?
Thank you.

Original post by LeaX
I don't know if too little sleep is taking its toll but I don't understand the answer to this question. Why is that Carbon chiral? I thought it needed four different groups bonded to it but that only has 3 groups (2 of which are the same as far as I can tell).
Does anyone know?
Thank you.

Does anyone know?
Thank you.

It's the skeletal formula so it doesn't show the hydrogen bond (which makes 4 bonds and then it has different ones everywhere else (the carbons either side are actually attached to different groups if your write the full formula/draw the structural formula out.
Original post by LeaX
I don't know if too little sleep is taking its toll but I don't understand the answer to this question. Why is that Carbon chiral? I thought it needed four different groups bonded to it but that only has 3 groups (2 of which are the same as far as I can tell).
Does anyone know?
Thank you.

Does anyone know?
Thank you.

It doesn't necessarily have to be 4 different elements, as long as the groups attached are different.
This means that if you have a ring, unless it has 2 Hydrogen's attached to it then it is likely that most of the carbons will be chiral.
So with this carbon, if you go 2 carbons left from the chiral one you get to a c=c double bond, but going two bonds to the right you get a c=o double bond. This makes these two groups different.
Unless going clockwise and anticlockwise from the the carbon gives exactly the same groups then the carbon is chiral.
See if you can get all the chiral centres on cholesterol, if you can then you'll have understood what I meant:
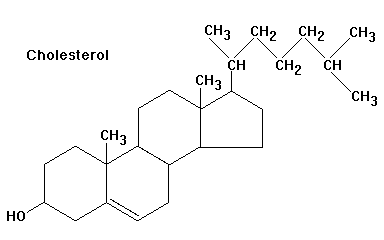
Spoiler
As always, chemguide explains it well:
http://www.chemguide.co.uk/basicorg/isomerism/optical.html
(edited 10 years ago)
Original post by senz72
Hi mate, I was wondering how is it all going and what are your plans for chemistry. What are you aiming for? What do you need to get the grade? If you did unit 1, how did it go? 

Not going as well as I planned (yet again) unfortunately, thanks for asking

Definitely screwed up unit 1... so I'll be keeping my 88 UMS from last year.
I quite literally need 84 UMS across my next 3 chemistry units now (2,4 & 5) in order to obtain my B grade

How's everything going with yourself ?
Original post by JRP95
It's the skeletal formula so it doesn't show the hydrogen bond (which makes 4 bonds and then it has different ones everywhere else (the carbons either side are actually attached to different groups if your write the full formula/draw the structural formula out.
Thank you.

Original post by GeorgeL3
It doesn't necessarily have to be 4 different elements, as long as the groups attached are different.
This means that if you have a ring, unless it has 2 Hydrogen's attached to it then it is likely that most of the carbons will be chiral.
So with this carbon, if you go 2 carbons left from the chiral one you get to a c=c double bond, but going two bonds to the right you get a c=o double bond. This makes these two groups different.
Unless going clockwise and anticlockwise from the the carbon gives exactly the same groups then the carbon is chiral.
See if you can get all the chiral centres on cholesterol, if you can then you'll have understood what I meant:

As always, chemguide explains it well:
http://www.chemguide.co.uk/basicorg/isomerism/optical.html
This means that if you have a ring, unless it has 2 Hydrogen's attached to it then it is likely that most of the carbons will be chiral.
So with this carbon, if you go 2 carbons left from the chiral one you get to a c=c double bond, but going two bonds to the right you get a c=o double bond. This makes these two groups different.
Unless going clockwise and anticlockwise from the the carbon gives exactly the same groups then the carbon is chiral.
See if you can get all the chiral centres on cholesterol, if you can then you'll have understood what I meant:

Spoiler
As always, chemguide explains it well:
http://www.chemguide.co.uk/basicorg/isomerism/optical.html
Thank you.
 The link you posted was really helpful too. I always forget about chemguide but it's such a good website.
The link you posted was really helpful too. I always forget about chemguide but it's such a good website.Hey  does anyone have or know where to find a soft copy of the CGP guide for A2 chem?
does anyone have or know where to find a soft copy of the CGP guide for A2 chem?
 does anyone have or know where to find a soft copy of the CGP guide for A2 chem?
does anyone have or know where to find a soft copy of the CGP guide for A2 chem?Original post by posthumus
Not going as well as I planned (yet again) unfortunately, thanks for asking 
Definitely screwed up unit 1... so I'll be keeping my 88 UMS from last year.
I quite literally need 84 UMS across my next 3 chemistry units now (2,4 & 5) in order to obtain my B grade
How's everything going with yourself ?

Definitely screwed up unit 1... so I'll be keeping my 88 UMS from last year.
I quite literally need 84 UMS across my next 3 chemistry units now (2,4 & 5) in order to obtain my B grade

How's everything going with yourself ?
Ah man, I'm sure you did well.
Struggling to revise though. I need to get 93 average in units 2,4 & 5 to get an A which is never going to happen so I guess it's a B for me (need to get 49 ums.)
Posted from TSR Mobile
I know the answer is B because when you oxidise alcohol dichromate goes orange to green(cr3+) but i also thought cr3+ is purple when hydrated?
Original post by Knoyle quiah
I know the answer is B because when you oxidise alcohol dichromate goes orange to green(cr3+) but i also thought cr3+ is purple when hydrated?
Yes, the answer is B. Chemguide offers this as an explaination:
The hexaaquachromium(III) ion is a "difficult to describe" violet-blue-grey
colour. However, when it is produced during a reaction in a test tube, it is
often green.
We nearly always describe the green ion as being
Cr3+(aq) - implying the hexaaquachromium(III) ion. That's
actually an over-simplification.
What happens is that one or more of the ligand water molecules get replaced
by a negative ion in the solution - typically sulphate or chloride.
Original post by Knoyle quiah
How do i work this one out?
You need to write out the two half equations, balance them so the number of electrons on each side are equal, then combine them to work out the ratios.
Hi sorry to requote you, but did you manage to think of my question ?
Thank you do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
Original post by Gnome :)
So if you have a burette that measures to 0.1cm3, you uncertainty is +/- 0.05, so 0.05*2=0.1 

Thank you
 do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?Original post by Gnome :)
Yes, the answer is B. Chemguide offers this as an explaination:
The hexaaquachromium(III) ion is a "difficult to describe" violet-blue-grey
colour. However, when it is produced during a reaction in a test tube, it is
often green.
We nearly always describe the green ion as being
Cr3+(aq) - implying the hexaaquachromium(III) ion. That's
actually an over-simplification.
What happens is that one or more of the ligand water molecules get replaced
by a negative ion in the solution - typically sulphate or chloride.
You need to write out the two half equations, balance them so the number of electrons on each side are equal, then combine them to work out the ratios.
The hexaaquachromium(III) ion is a "difficult to describe" violet-blue-grey
colour. However, when it is produced during a reaction in a test tube, it is
often green.
We nearly always describe the green ion as being
Cr3+(aq) - implying the hexaaquachromium(III) ion. That's
actually an over-simplification.
What happens is that one or more of the ligand water molecules get replaced
by a negative ion in the solution - typically sulphate or chloride.
You need to write out the two half equations, balance them so the number of electrons on each side are equal, then combine them to work out the ratios.
OK thanks

Original post by jojo1995
Hi sorry to requote you, but did you manage to think of my question ?
Thank you do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
Thank you
 do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?
do you know what the percentage error for a pipette or a thermometer would be ? How you would calculate it ?Look at how many decimal places the instrument records to. I don't really know how to word it, but if it's a whole number, it can be 0.5 either side. 1dp, 0.05 either side, 2dp 0.005 either side.... Do you kind of get what I mean? That's the uncertaincy.
Then you just follow the same as before
 (uncertainty x 2/measurement all times by 100 to get the percentage)
(uncertainty x 2/measurement all times by 100 to get the percentage)Original post by Gnome :)
Look at how many decimal places the instrument records to. I don't really know how to word it, but if it's a whole number, it can be 0.5 either side. 1dp, 0.05 either side, 2dp 0.005 either side.... Do you kind of get what I mean? That's the uncertaincy.
Then you just follow the same as before (uncertainty x 2/measurement all times by 100 to get the percentage)
(uncertainty x 2/measurement all times by 100 to get the percentage)
Then you just follow the same as before
 (uncertainty x 2/measurement all times by 100 to get the percentage)
(uncertainty x 2/measurement all times by 100 to get the percentage)ahh i see, thank you
 do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?
do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?Original post by jojo1995
ahh i see, thank you  do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?
do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?
 do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?
do you *2 for a pipette and a thermometer too ? dont we only take one reading with a pipette ?You *2 because the real value could be 0.5 or whatever above or below the recorded value
Does anyone have any resources for the organic synthesis section? Or any tips on how to revise it? Really not sure what I'm supposed to know in what depth, since we had to rush it in school 

Original post by AtomicMan
Does anyone have any resources for the organic synthesis section? Or any tips on how to revise it? Really not sure what I'm supposed to know in what depth, since we had to rush it in school 

I have basic reaction schema for the organic and aromatic stuff where you have to fill out the reactants/conditions, would you like those?
Original post by HarryMWilliams
I have basic reaction schema for the organic and aromatic stuff where you have to fill out the reactants/conditions, would you like those?
Thanks, but the bit Im struggling with is all the techniques like filteration, recrystillisation etc and combinotorial chemistry
Posted from TSR Mobile
Original post by AtomicMan
Thanks, but the bit Im struggling with is all the techniques like filteration, recrystillisation etc and combinotorial chemistry
Posted from TSR Mobile
Posted from TSR Mobile
I see, in that case, I'd recommend ploughing through ChemGuide, there is a lot of useful information on there which I have personally found helpful.
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- IAL repeats cash in.
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- Over 500 questions on AQA Bio Unit 4 + Current Spec and old Spec papers + MS!
- Self-teaching Chemistry A-level (as a private candidate)?
- Official thread - May/June 2023 Edexcel IAL
- Switching from A level to IAL
- A-level Biology Study Group 2022-2023
- Trek To The End Of Year 13 ❀◕ ‿ ◕❀
- Uniq 2023
- School is killing me - Y11 "GYG" 2022
- BTEC Results FINAL Certificate
- Revision Struggles?! Join the 2023 TSR All Day Revision Thread!
- Best timetable for studying 4 subjects?
- Edexcel A-Level French Paper 2 (9FR0 02) - 19th June 2024 [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- A-level Chemistry Study Group 2022-2023
- iria's 'grow your grades' blog :)
Latest
Trending
Last reply 22 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 22 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



