This discussion is now closed.
Check out other Related discussions
- A-level Exam Discussions 2024
- Help polymers
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- AQA A level Chemistry June 2022 Papers
- OCR A-LEVEL CHEMISTRY A (H432/03) - 21st June [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Does UCL accept Human biology A level ?
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]
- OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]
- OCR A-Level Chemistry B Paper 1 (H433/01) - 12th June 2023 [Exam Chat]
- A-level Chemistry Study Group 2022-2023
- A Level Advice
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Last minute chemistry revision advice
- 2nd Alevel RETAKE ...
OCR F324-June 2013 Chemistry
Scroll to see replies
I reckon there will be a big combined techniques question at the end. Hopefully there is 

Original post by D4rth
If its more soluble in the stationary phase it has a longer time as its harder for the mobile phase to carry it through the column.
It depends on what the greater solubility is in, if its more soluble in the mobile phase retention time is shorter.
Posted from TSR Mobile
It depends on what the greater solubility is in, if its more soluble in the mobile phase retention time is shorter.
Posted from TSR Mobile
thanks could you explain for both tlc and gas
is tlc always adsorption?
in tlc if its more soluble/adsorpable(?) in stationary phase will rf value be greater, same with gas chromotography retention time greater
Original post by thers
One more
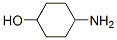
How many chiral centres in this molecule? [1]

How many chiral centres in this molecule? [1]
Is it 1?
Edit: I saw your answer
Original post by thers
One more

How many chiral centres in this molecule? [1]

How many chiral centres in this molecule? [1]
is there 0?
Original post by otrivine
why do you have blue and red? do they mean separate dont get why they are coloured
Lol I just randomly chose this molecule from google images - I don't know why they are coloured (probably to show the distinct functional groups.
Can anyone help me with this?
Describe and explain the increased use of esters of fatty acids as biodiesel.
Describe and explain the increased use of esters of fatty acids as biodiesel.
Original post by thers
Answer
Spoiler
Why is it 0?
Posted from TSR Mobile
Original post by D4rth
NH and OH always appear as singlets.
Watch this video
https://www.youtube.com/watch?v=k6F__pysc74&feature=youtube_gdata_player
Posted from TSR Mobile
Watch this video
https://www.youtube.com/watch?v=k6F__pysc74&feature=youtube_gdata_player
Posted from TSR Mobile
that video is amazing but how does he know how big to make the peaks when he draws them? Thanks.
Original post by AFC_123456789
that video is amazing but how does he know how big to make the peaks when he draws them? Thanks.
They're just relative, the peak with 3 protons in the environment will be bigger than the peak with 2 protons in the environment. I don't think the examiners will be that picky though.
Posted from TSR Mobile
Any predictions on what might come up
Original post by Varsh05
Can anyone help me with this?
Describe and explain the increased use of esters of fatty acids as biodiesel.
Describe and explain the increased use of esters of fatty acids as biodiesel.
Biodegradable - breakdown doesn't emit harmful gases.
Made form plants so carbon neutral/environmentally friendly.
How many marks is it?
Original post by D4rth
I assume you mean Jan 13? Its been posted many times, check the last few pages, it'll be in an attachment.
Posted from TSR Mobile
Posted from TSR Mobile
yeah my bad!! thanks
Original post by D4rth
Lets look at the carbon with the NH2 group sticking out of it. The groups coming out are -NH2, -H, -CH2CH2CHOH and -CH2CH2CHOH. Same can be said for the carbon with OH sticking out but swap the OH with NH2. So those 2 carbons are not chiral
Original post by _HabibaH_
Biodegradable - breakdown doesn't emit harmful gases.
Made form plants so carbon neutral/environmentally friendly.
How many marks is it?
Made form plants so carbon neutral/environmentally friendly.
How many marks is it?
It's just a question from the spec that we're supposed to know.
Just did Jan 13 - scored 85 and in Jan I just scraped a C. I don't know whether to be crazy happy or crazy confused.
Just me or is it anyone else's last exam and I've lost all motivation
HELP
HELP
Original post by _HabibaH_
Just did Jan 13 - scored 85 and in Jan I just scraped a C. I don't know whether to be crazy happy or crazy confused.
Is that 85 UMS lol?
Original post by thers
Lets look at the carbon with the NH2 group sticking out of it. The groups coming out are -NH2, -H, -CH2CH2CHOH and -CH2CH2CHOH. Same can be said for the carbon with OH sticking out but swap the OH with NH2. So those 2 carbons are not chiral
Oh dammit, I'm used to more complex molecules where the things on either side of the chain are completely different. Thanks
Posted from TSR Mobile
Original post by _HabibaH_
Just did Jan 13 - scored 85 and in Jan I just scraped a C. I don't know whether to be crazy happy or crazy confused.
Jan 13 was a bitch of a paper did it for our mock got a C or something dismal
Original post by livvy965
Just me or is it anyone else's last exam and I've lost all motivation
HELP
HELP
It's mine as well and I had maths this morning and really just can't concentrate. I'm downing coffee and coke trying to get as much caffeine as possible

Related discussions
- A-level Exam Discussions 2024
- Help polymers
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR A GCSE Chemistry Paper 2 (Foundation Combined) J250/04- 13th June [Exam Chat]
- AQA A level Chemistry June 2022 Papers
- OCR A-LEVEL CHEMISTRY A (H432/03) - 21st June [Exam Chat]
- Using Old Spec to Revise New Spec (Maths, Chemistry, Biology) A level
- Does UCL accept Human biology A level ?
- OCR A-Level Chemistry B Paper 3 (H433/03) - 23rd June 2023 [Exam Chat]
- OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]
- OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]
- OCR A-Level Chemistry B Paper 1 (H433/01) - 12th June 2023 [Exam Chat]
- A-level Chemistry Study Group 2022-2023
- A Level Advice
- OCR A GCSE Chemistry Paper 1 (Foundation Combined) J250/03- 22nd May 2023 [Exam Chat]
- OCR A GCSE Chemistry Paper 1 (Higher Combined) J250/09- 22nd May 2023 [Exam Chat]
- Last minute chemistry revision advice
- 2nd Alevel RETAKE ...
Latest
Trending
Last reply 11 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 11 hours ago
OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 2 weeks ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 Foundation Triple (8462 1F) - 17th May 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



