Calculations
One form of the Van’t Hoff isotherm is:
1)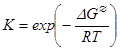
where K is the equilibrium constant, ΔG∅ the standard change in Gibbs free energy, R the gas constant, and T the absolute temperature.
Determine the value of K when ΔG∅ = 2.0 kJmol-1, with R = 8.314 JK-1mol-1, and T = 0°C. Use the value of 273.15 where appropriate.
Give your answer to 3 d.p.
Answer
for this question I am getting the answer to be 0.415
1)
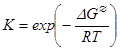
where K is the equilibrium constant, ΔG∅ the standard change in Gibbs free energy, R the gas constant, and T the absolute temperature.
Determine the value of K when ΔG∅ = 2.0 kJmol-1, with R = 8.314 JK-1mol-1, and T = 0°C. Use the value of 273.15 where appropriate.
Give your answer to 3 d.p.
Answer
for this question I am getting the answer to be 0.415
Hi there,
While you're waiting for an answer, did you know we have 300,000 study resources that could answer your question in TSR's Learn together section?
We have everything from Teacher Marked Essays to Mindmaps and Quizzes to help you with your work. Take a look around.
If you're stuck on how to get started, try creating some resources. It's free to do and can help breakdown tough topics into manageable chunks. Get creating now.
Thanks!
Not sure what all of this is about? Head here to find out more.
While you're waiting for an answer, did you know we have 300,000 study resources that could answer your question in TSR's Learn together section?
We have everything from Teacher Marked Essays to Mindmaps and Quizzes to help you with your work. Take a look around.
If you're stuck on how to get started, try creating some resources. It's free to do and can help breakdown tough topics into manageable chunks. Get creating now.
Thanks!
Not sure what all of this is about? Head here to find out more.
Original post by Lufthansa
One form of the Van’t Hoff isotherm is:
1)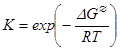
where K is the equilibrium constant, ΔG∅ the standard change in Gibbs free energy, R the gas constant, and T the absolute temperature.
Determine the value of K when ΔG∅ = 2.0 kJmol-1, with R = 8.314 JK-1mol-1, and T = 0°C. Use the value of 273.15 where appropriate.
Give your answer to 3 d.p.
Answer
for this question I am getting the answer to be 0.415
1)
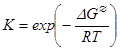
where K is the equilibrium constant, ΔG∅ the standard change in Gibbs free energy, R the gas constant, and T the absolute temperature.
Determine the value of K when ΔG∅ = 2.0 kJmol-1, with R = 8.314 JK-1mol-1, and T = 0°C. Use the value of 273.15 where appropriate.
Give your answer to 3 d.p.
Answer
for this question I am getting the answer to be 0.415
I get that too. Is there a problem?
Quick Reply
Related discussions
- Calculator for a level maths
- Am I required to write the calculator model name on my paper?
- Exam mode settings
- Fx-cg50 not showing answers in surd form..
- Am I allowed a Calculator for university Computer Science exams?
- Btec grade calculation
- PAT 2023 Online Calculator
- GCSE Allowed Calculators Help
- how to revise for maths a-level
- Casio fx-cg50
- Calculator
- Calculator not showing exact answers
- Calculator not giving exact answers
- How much to use calculator in Further Stats?
- A level physics question on gravitational fields
- Edexcel old spec papers (maths)
- Calculator help
- FX-CG50 Worth It?
- a level maths
- Using the Graphical Calculator (CASIO FX-CG50) for A Level Physics (OCR A)
Latest
Trending
Last reply 2 days ago
Did Cambridge maths students find maths and further maths a level very easy?Last reply 2 weeks ago
Edexcel A Level Mathematics Paper 2 unofficial mark scheme correct me if wrongMaths
71
Trending
Last reply 2 days ago
Did Cambridge maths students find maths and further maths a level very easy?Last reply 2 weeks ago
Edexcel A Level Mathematics Paper 2 unofficial mark scheme correct me if wrongMaths
71




