Mass Defect and Binding Energies
Hi, this whole topic has eluded me for an entire week - it's time to come to you guys.
i can't make the link between energy being released and atoms fissioning/fusion depending on their relative position to iron.
I cant ant give anything more detailed than that because i simply don't what's going on here!
i can't make the link between energy being released and atoms fissioning/fusion depending on their relative position to iron.
I cant ant give anything more detailed than that because i simply don't what's going on here!
Scroll to see replies
It doesn't really help us to help you if you don't give us some idea of what you already know.
Have you ever seen this graph?
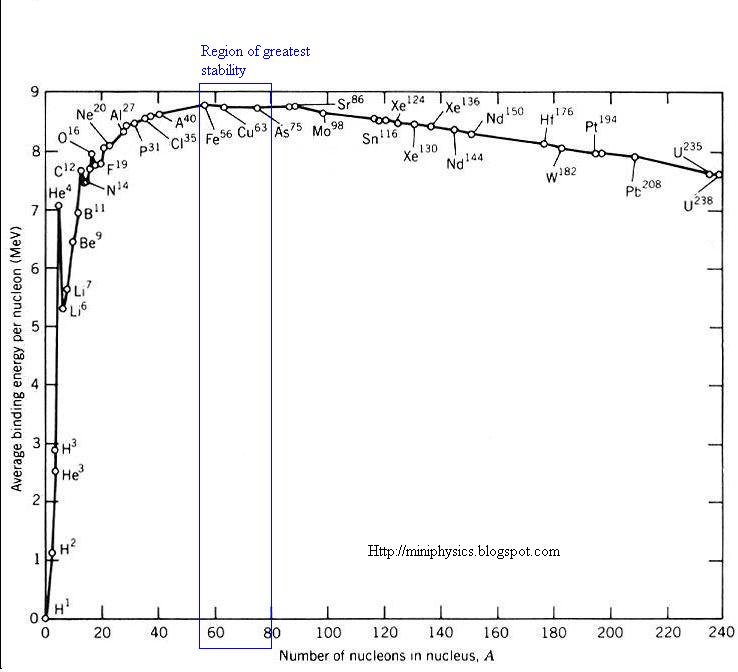
Have you ever seen this graph?
Original post by Stonebridge
It doesn't really help us to help you if you don't give us some idea of what you already know.
Have you ever seen this graph?

Have you ever seen this graph?
Original post by genuinelydense
no i haven't. All I know is that energy is released when things less massive than iron fuse together, and that that's something to do with binding energies (which I don't know much about either).
If you don't know what nuclear binding energy is and you have never seen that graph then I can only conclude you haven't been taught this topic.
This isn't the place to learn it or for us to try to teach you it.
What have you done on fission/fusion in class?
Do you have a specific exam question you are trying to answer?
Original post by Stonebridge
If you don't know what nuclear binding energy is and you have never seen that graph then I can only conclude you haven't been taught this topic.
This isn't the place to learn it or for us to try to teach you it.
What have you done on fission/fusion in class?
Do you have a specific exam question you are trying to answer?
This isn't the place to learn it or for us to try to teach you it.
What have you done on fission/fusion in class?
Do you have a specific exam question you are trying to answer?
you're right. i'll leave this thread here until i know more about it (asking teacher today).
Original post by Stonebridge
If you don't know what nuclear binding energy is and you have never seen that graph then I can only conclude you haven't been taught this topic.
This isn't the place to learn it or for us to try to teach you it.
What have you done on fission/fusion in class?
Do you have a specific exam question you are trying to answer?
This isn't the place to learn it or for us to try to teach you it.
What have you done on fission/fusion in class?
Do you have a specific exam question you are trying to answer?
Alright, I've learned more facts about the phenomenon and can now, hopefully, articulate my concerns.
My problems are centred around kinetic energy being 'let out' by particles when they either combine or split up to make elements with higher binding energies. All I really follow is that when - for whatever reason - elements with higher binding energies are formed, the overall mass of the system decreases.
(edited 10 years ago)
Original post by genuinelydense
Alright, I've learned more facts about the phenomenon and can now, hopefully, articulate my concerns.
My problems are centred around kinetic energy being 'let out' by particles when they either combine or split up to make elements with higher binding energies. All I really follow is that when - for whatever reason - elements with higher binding energies are formed, the overall mass of the system decreases.
My problems are centred around kinetic energy being 'let out' by particles when they either combine or split up to make elements with higher binding energies. All I really follow is that when - for whatever reason - elements with higher binding energies are formed, the overall mass of the system decreases.
It's not so much about kinetic energy being "let out", it's about the fact that if you have two or more particles that are subject to an attractive force (in this case the nuclear forces) then when they get closer together they lose energy. It's actually potential energy. Just in the same way as a mass that falls to the Earth loses potential energy.
The second point is that energy can be turned into mass and mass into energy.
Have you studied E=mc2?
In a nucleus, the loss in energy when the nucleus forms from all the constituent parts manifests itself as a loss in mass.
The final nucleus always has less mass than the sum of the masses of the constituent parts.
This lost mass (or energy) is what is called the binding energy of the nucleus.
It's called this because, in order to separate the nucleus back into its constituent particles you need to do work to put this energy back - work is done against the strong attractive forces holding the nucleus together. This work is equal to the binding energy.
Have you done this theory in class?
(edited 10 years ago)
Original post by Stonebridge
It's not so much about kinetic energy being "let out", it's about the fact that if you have two or more particles that are subject to an attractive force (in this case the nuclear forces) then when they get closer together they lose energy. It's actually potential energy. Just in the same way as a mass that falls to the Earth loses potential energy.
The second point is that energy can be turned into mass and mass into energy.
Have you studied E=mc2?
In a nucleus, the loss in energy when the nucleus forms from all the constituent parts manifests itself as a loss in mass.
The final nucleus always has less mass than the sum of the masses of the constituent parts.
This lost mass (or energy) is what is called the binding energy of the nucleus.
It's called this because, in order to separate the nucleus back into its constituent particles you need to do work to put this energy back - work is done against the strong attractive forces holding the nucleus together. This work is equal to the binding energy.
Have you done this theory in class?
The second point is that energy can be turned into mass and mass into energy.
Have you studied E=mc2?
In a nucleus, the loss in energy when the nucleus forms from all the constituent parts manifests itself as a loss in mass.
The final nucleus always has less mass than the sum of the masses of the constituent parts.
This lost mass (or energy) is what is called the binding energy of the nucleus.
It's called this because, in order to separate the nucleus back into its constituent particles you need to do work to put this energy back - work is done against the strong attractive forces holding the nucleus together. This work is equal to the binding energy.
Have you done this theory in class?
Yes, learned about e=mc^2 today. We also learned about nuclear reactors.
Why is it incorrect to say that they have let energy out as kinetic energy? What you've said has confused me somewhat.. I understand that the nuclei have some form of negative energy due to position: it would require work to be done to push the two nuclei together, due to the Coulomb forces between them. So I understand that they're losing energy by getting close to each other, but what does this have to do with loss in mass? Furthermore, what does this have to do with massive energy release that the nuclear power station can harness? I'm sorry, I'm just not connecting up the dots. :/
It seems to me that you still haven't studied this topic completely. Maybe you've seen some past paper questions and are trying to find out how to answer them.
I strongly recommend you wait until you have finished this topic in class before attempting to understand it all. If you only have part of the picture you are not going to understand topics that require you have learned the whole picture.
Have you looked at that graph I posted yet in class? You are not going to get much further until you have.
Well I didn't say it was incorrect.
I said "It's not so much about kinetic energy being "let out", it's about ..."
And then tried to explain what it is about.
I understand that the nuclei have some form of negative energy due to position: it would require work to be done to push the two nuclei together, due to the Coulomb forces between them.
Yes, but this has nothing to do with nuclear binding energy.
So I understand that they're losing energy by getting close to each other
No. You don't understand. If it was about pushing them together against Coulomb forces the particles would gain (potential) energy, not lose it. The energy lost by creating a nucleus from its constituents is due to the strong attractive (nuclear, not Coulomb) forces that keep the nucleus together.
but what does this have to do with loss in mass?
I explained this in my last post. E=mc2
Furthermore, what does this have to do with massive energy release that the nuclear power station can harness? I'm sorry, I'm just not connecting up the dots. :/
You're not connecting them because you don't have them all there yet. You need to have studied that graph in my first post. Energy is released when there is an overall loss in energy when a nucleus forms. In a fusion reactor the fragment nuclei that form after the split have less energy in total than the single nucleus that they formed from. This energy is released (compare exothermic in chemistry) most in the form of the kinetic energy of the fragments.
You need to have studied that graph and how the binding energy of the various element nuclei varies with nucleon number.
Have you done this yet?
I strongly recommend you wait until you have finished this topic in class before attempting to understand it all. If you only have part of the picture you are not going to understand topics that require you have learned the whole picture.
Have you looked at that graph I posted yet in class? You are not going to get much further until you have.
Original post by genuinelydense
Yes, learned about e=mc^2 today. We also learned about nuclear reactors.
Why is it incorrect to say that they have let energy out as kinetic energy? What you've said has confused me somewhat
Why is it incorrect to say that they have let energy out as kinetic energy? What you've said has confused me somewhat
Well I didn't say it was incorrect.

I said "It's not so much about kinetic energy being "let out", it's about ..."
And then tried to explain what it is about.
I understand that the nuclei have some form of negative energy due to position: it would require work to be done to push the two nuclei together, due to the Coulomb forces between them.
Yes, but this has nothing to do with nuclear binding energy.
So I understand that they're losing energy by getting close to each other
No. You don't understand. If it was about pushing them together against Coulomb forces the particles would gain (potential) energy, not lose it. The energy lost by creating a nucleus from its constituents is due to the strong attractive (nuclear, not Coulomb) forces that keep the nucleus together.
but what does this have to do with loss in mass?
I explained this in my last post. E=mc2
Furthermore, what does this have to do with massive energy release that the nuclear power station can harness? I'm sorry, I'm just not connecting up the dots. :/
You're not connecting them because you don't have them all there yet. You need to have studied that graph in my first post. Energy is released when there is an overall loss in energy when a nucleus forms. In a fusion reactor the fragment nuclei that form after the split have less energy in total than the single nucleus that they formed from. This energy is released (compare exothermic in chemistry) most in the form of the kinetic energy of the fragments.
You need to have studied that graph and how the binding energy of the various element nuclei varies with nucleon number.
Have you done this yet?
Original post by Stonebridge
It seems to me that you still haven't studied this topic completely. Maybe you've seen some past paper questions and are trying to find out how to answer them.
I strongly recommend you wait until you have finished this topic in class before attempting to understand it all. If you only have part of the picture you are not going to understand topics that require you have learned the whole picture.
Have you looked at that graph I posted yet in class? You are not going to get much further until you have.
Well I didn't say it was incorrect.
I said "It's not so much about kinetic energy being "let out", it's about ..."
And then tried to explain what it is about.
Yes, but this has nothing to do with nuclear binding energy.
No. You don't understand. If it was about pushing them together against Coulomb forces the particles would gain (potential) energy, not lose it. The energy lost by creating a nucleus from its constituents is due to the strong attractive (nuclear, not Coulomb) forces that keep the nucleus together.
I explained this in my last post. E=mc2
You're not connecting them because you don't have them all there yet. You need to have studied that graph in my first post. Energy is released when there is an overall loss in energy when a nucleus forms. In a fusion reactor the fragment nuclei that form after the split have less energy in total than the single nucleus that they formed from. This energy is released (compare exothermic in chemistry) most in the form of the kinetic energy of the fragments.
You need to have studied that graph and how the binding energy of the various element nuclei varies with nucleon number.
Have you done this yet?
I strongly recommend you wait until you have finished this topic in class before attempting to understand it all. If you only have part of the picture you are not going to understand topics that require you have learned the whole picture.
Have you looked at that graph I posted yet in class? You are not going to get much further until you have.
Well I didn't say it was incorrect.

I said "It's not so much about kinetic energy being "let out", it's about ..."
And then tried to explain what it is about.
Yes, but this has nothing to do with nuclear binding energy.
No. You don't understand. If it was about pushing them together against Coulomb forces the particles would gain (potential) energy, not lose it. The energy lost by creating a nucleus from its constituents is due to the strong attractive (nuclear, not Coulomb) forces that keep the nucleus together.
I explained this in my last post. E=mc2
You're not connecting them because you don't have them all there yet. You need to have studied that graph in my first post. Energy is released when there is an overall loss in energy when a nucleus forms. In a fusion reactor the fragment nuclei that form after the split have less energy in total than the single nucleus that they formed from. This energy is released (compare exothermic in chemistry) most in the form of the kinetic energy of the fragments.
You need to have studied that graph and how the binding energy of the various element nuclei varies with nucleon number.
Have you done this yet?
Alright, I think I'm getting somewhere.
So after they've overcome the coulomb forces, they reach the strong nuclear force's effective range. The situation is then sorta comparable to someone free-falling under Earth's gravity - potential is lost. Although I don't understand how this releases energy: they reach a range at which they're attracted to each other, but will then 'collide' (reach the nuclear forces repulsive range) and then stay together. I mean, what exactly is the mechanism through which the now combined nuclei begin to travel at a much greater velocity?
I think I 'understand' the thing to do with the binding energy graph... they're sort of like bond enthalpies, right? High bond energy/binding energy = more work needed to pull them apart, or, through conservation of energy, the energy that's released when the bonds are formed.
I get all of that stuff in a kind of 'A-level' way, but not properly/intuitively. Thanks for being so patient btw; I find it hard to articulate myself online.
(edited 10 years ago)
Original post by genuinelydense
Alright, I think I'm getting somewhere.
So after they've overcome the coulomb forces, they reach the strong nuclear force's effective range. The situation is then sorta comparable to someone free-falling under Earth's gravity - potential is lost. Although I don't understand how this releases energy: they reach a range at which they're attracted to each other, but will then 'collide' (reach the nuclear forces repulsive range) and then stay together. I mean, what exactly is the mechanism through which the now combined nuclei begin to travel at a much greater velocity?
I think I 'understand' the thing to do with the binding energy graph... they're sort of like bond enthalpies, right? High bond energy/binding energy = more work needed to pull them apart, or, through conservation of energy, the energy that's released when the bonds are formed.
I get all of that stuff in a kind of 'A-level' way, but not properly/intuitively. Thanks for being so patient btw; I find it hard to articulate myself online.
So after they've overcome the coulomb forces, they reach the strong nuclear force's effective range. The situation is then sorta comparable to someone free-falling under Earth's gravity - potential is lost. Although I don't understand how this releases energy: they reach a range at which they're attracted to each other, but will then 'collide' (reach the nuclear forces repulsive range) and then stay together. I mean, what exactly is the mechanism through which the now combined nuclei begin to travel at a much greater velocity?
I think I 'understand' the thing to do with the binding energy graph... they're sort of like bond enthalpies, right? High bond energy/binding energy = more work needed to pull them apart, or, through conservation of energy, the energy that's released when the bonds are formed.
I get all of that stuff in a kind of 'A-level' way, but not properly/intuitively. Thanks for being so patient btw; I find it hard to articulate myself online.
You'll find as you do more advanced physics that, unfortunately, it is often not possible to understand it intuitively.
The best I can suggest at the moment it to wait until you have to do a past paper question on this topic (after doing all the required theory in class) and ask for help here if you have any difficulties with it.
Original post by Stonebridge
You'll find as you do more advanced physics that, unfortunately, it is often not possible to understand it intuitively.
No way. A-level physics is the most unintuitive farce of a subject ever. They bung in a little bit of everything but dumb it down so more kids can understand, no, memorise it.
OP the right hand side of the graph is the easiest bit to understand so look at that first. When atoms are pulled apart, you can consider the excess energy as 'shrapnel' from the process. Look up nuclear fission online to get some good diagrams showing how the energy is released in chain reactions. For the left hand side of the graph, it may help if you get on Wikipedia and look up the P-P chain or C-N-O chain of fusion in stars. The diagrams there may give a better idea of where the excess energy comes from.
Posted from TSR Mobile
Original post by Pessimisterious
"No way" what? Are you agreeing or disagreeing that it's not intuitive?
OP the right hand side of the graph is the easiest bit to understand so look at that first. When atoms are pulled apart, you can consider the excess energy as 'shrapnel' from the process.
I think we can all think intuitively about things flying apart and shrapnel. That's not quite the point. That's a good analogy to describe what happens physically.
The poster is not asking for that to be intuitive, she is asking why there is "excess energy" in the first place. Do you have an "intuitive" answer to that? I'd be pleased to hear it and use it in the future when asked the same question again.
Original post by Stonebridge
"No way" what? Are you agreeing or disagreeing that it's not intuitive?
I think we can all think intuitively about things flying apart and shrapnel. That's not quite the point. That's a good analogy to describe what happens physically.
The poster is not asking for that to be intuitive, she is asking why there is "excess energy" in the first place. Do you have an "intuitive" answer to that? I'd be pleased to hear it and use it in the future when asked the same question again.
I think we can all think intuitively about things flying apart and shrapnel. That's not quite the point. That's a good analogy to describe what happens physically.
The poster is not asking for that to be intuitive, she is asking why there is "excess energy" in the first place. Do you have an "intuitive" answer to that? I'd be pleased to hear it and use it in the future when asked the same question again.
Well, you said that as physics gets more advanced it becomes more difficult to understand intuitively. I disagree because I think a lot of things are far better understood when taken beyond the scope of A-level teaching. So things like nuclear fission energy, and a lot of other tripe that they bung into the A-level syllabus, are too lightly skimmed over for it to be really understood. They're trying to make it easier, but it seems to be that it's only 'easier' if you simply memorise the right bits to pass the exams. Hence people like the OP asking for more info, because the way it's presented at sixth form is far too watered down for it to make any sense at all.
At undergrad level I think stuff gets more intuitive because you gain a much deeper grasp on the real workings of all the processes. Honestly I can't believe how much of a bodge the current general physics A-level syllabus is. So much info with no explanation. Hell, I don't even know why they bother teaching physics to students who aren't taking maths as well. Something to do with 'accessibility', I guess. Sorry, bit of a rant, not aimed at you, heh.
In general I just disagree that physics gets less intuitive at more advanced stages. I find it far easier now than I did at A-level.
Anyway.
May or may not be helpful but this is as much as I can add:
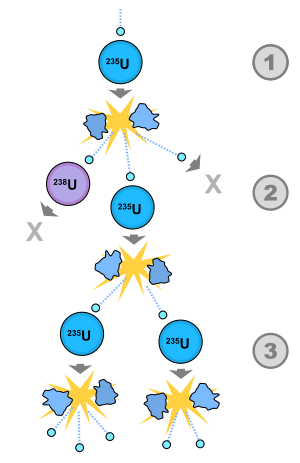
Fission chain reaction. The U235 breaks into two smaller atoms. The force/energy holding the smaller atoms together is less than the force/energy that held together the U235. The energy must still exist, it can't just become nothing. So it is emitted and dissipated in it's final form - heat.

Fusion chain reaction. The sort of explodey bits are representative of the excess energy of the reaction. Two atoms come together to form a new atom that is lighter than the mass of the original two. If the new atom is lighter, then... where did that extra mass go? It was ejected as pure energy.
As you already said, OP needs a better grasp of E=mc2 to properly understand how this can be the case, but, as I said, it's hard to get to grips with such a concept when the syllabus does nothing but lightly state the formula and scoot on to the next chapter!
Original post by Pessimisterious

Fission chain reaction. The U235 breaks into two smaller atoms. The force/energy holding the smaller atoms together is less than the force/energy that held together the U235. The energy must still exist, it can't just become nothing. So it is emitted and dissipated in it's final form - heat.

Fission chain reaction. The U235 breaks into two smaller atoms. The force/energy holding the smaller atoms together is less than the force/energy that held together the U235. The energy must still exist, it can't just become nothing. So it is emitted and dissipated in it's final form - heat.
Thanks for the reply.
What's intuitive about the bold bit in your description above. Why is it less? Intuitively.
(edited 10 years ago)
Original post by Stonebridge
Thanks for the reply.
What's intuitive about the bold bit in your description above. Why is it less? Intuitively.
What's intuitive about the bold bit in your description above. Why is it less? Intuitively.
Heh, my bad, I kind of skimmed over that bit.
Change force for mass, and again it's explained pretty much the same way as before with the whole E=mc2 thing.
To be honest my aim wasn't to explain fusion to the OP, I just wanted to argue my point that lot of this stuff does, in my opinion, become more intuitive when you have a greater expanse of knowledge with which to understand it. And this only comes at levels beyond the scope of the A-level course.
I mean I could upload all my lecture notes on relativity and the logical derivation/proof of the relation between mass and energy for the OP to study for a few weeks, along with all the relevant calculus etc, and then maybe fusion and fission might make a little more logical/intuitive sense.
For most of my physics A-level I had to grit my teeth and comfort myself with the hope that undergrad level would 'explain all'. And so far it is doing just that! I presume OP has reached a similar kind of brick wall - being told something is true, yet none of the book explains exactly why it's true.
e.g. I think it's criminal that the A-level syllabus teaches of wave-particle duality and expects students to simply memorise, 'particles are like waves', and explain the proof of each case. It's hardly physics, it's just memorisation. Plenty of people must be utterly frustrated at the lack of reason behind thing things they get told.
Sorry this rant of mine seems to be going way off topic so I'll leave it now. Simply I want to say that I think physics does become more intuitive once you're given more information beyond the scope of the A-level farce. It's just an accumulation of logic that happens to currently be presented in entirely the wrong order.
Original post by genuinelydense
Yes, learned about e=mc^2 today. We also learned about nuclear reactors.
Why is it incorrect to say that they have let energy out as kinetic energy? What you've said has confused me somewhat.. I understand that the nuclei have some form of negative energy due to position: it would require work to be done to push the two nuclei together, due to the Coulomb forces between them. So I understand that they're losing energy by getting close to each other, but what does this have to do with loss in mass? Furthermore, what does this have to do with massive energy release that the nuclear power station can harness? I'm sorry, I'm just not connecting up the dots. :/
Why is it incorrect to say that they have let energy out as kinetic energy? What you've said has confused me somewhat.. I understand that the nuclei have some form of negative energy due to position: it would require work to be done to push the two nuclei together, due to the Coulomb forces between them. So I understand that they're losing energy by getting close to each other, but what does this have to do with loss in mass? Furthermore, what does this have to do with massive energy release that the nuclear power station can harness? I'm sorry, I'm just not connecting up the dots. :/
Sir, you are talking about two concepts it seems. One is fusion and one is fission, form what you have said, it seems that you have mixed up ideas from fission and fusion.
Original post by Pessimisterious
Heh, my bad, I kind of skimmed over that bit.
Change force for mass, and again it's explained pretty much the same way as before with the whole E=mc2 thing.
To be honest my aim wasn't to explain fusion to the OP, I just wanted to argue my point that lot of this stuff does, in my opinion, become more intuitive when you have a greater expanse of knowledge with which to understand it. And this only comes at levels beyond the scope of the A-level course.
I mean I could upload all my lecture notes on relativity and the logical derivation/proof of the relation between mass and energy for the OP to study for a few weeks, along with all the relevant calculus etc, and then maybe fusion and fission might make a little more logical/intuitive sense.
For most of my physics A-level I had to grit my teeth and comfort myself with the hope that undergrad level would 'explain all'. And so far it is doing just that! I presume OP has reached a similar kind of brick wall - being told something is true, yet none of the book explains exactly why it's true.
e.g. I think it's criminal that the A-level syllabus teaches of wave-particle duality and expects students to simply memorise, 'particles are like waves', and explain the proof of each case. It's hardly physics, it's just memorisation. Plenty of people must be utterly frustrated at the lack of reason behind thing things they get told.
Sorry this rant of mine seems to be going way off topic so I'll leave it now. Simply I want to say that I think physics does become more intuitive once you're given more information beyond the scope of the A-level farce. It's just an accumulation of logic that happens to currently be presented in entirely the wrong order.
Change force for mass, and again it's explained pretty much the same way as before with the whole E=mc2 thing.
To be honest my aim wasn't to explain fusion to the OP, I just wanted to argue my point that lot of this stuff does, in my opinion, become more intuitive when you have a greater expanse of knowledge with which to understand it. And this only comes at levels beyond the scope of the A-level course.
I mean I could upload all my lecture notes on relativity and the logical derivation/proof of the relation between mass and energy for the OP to study for a few weeks, along with all the relevant calculus etc, and then maybe fusion and fission might make a little more logical/intuitive sense.
For most of my physics A-level I had to grit my teeth and comfort myself with the hope that undergrad level would 'explain all'. And so far it is doing just that! I presume OP has reached a similar kind of brick wall - being told something is true, yet none of the book explains exactly why it's true.
e.g. I think it's criminal that the A-level syllabus teaches of wave-particle duality and expects students to simply memorise, 'particles are like waves', and explain the proof of each case. It's hardly physics, it's just memorisation. Plenty of people must be utterly frustrated at the lack of reason behind thing things they get told.
Sorry this rant of mine seems to be going way off topic so I'll leave it now. Simply I want to say that I think physics does become more intuitive once you're given more information beyond the scope of the A-level farce. It's just an accumulation of logic that happens to currently be presented in entirely the wrong order.
It's ok to have a rant.

I think where we disagree is that we have slightly differing definitions of "intuitive".
Of course physics makes more sense the more you learn. The various bits start to fit together and you can begin to see patterns and connections. I don't disagree with that.
Thanks for taking the time to discuss this with me.
Original post by Pessimisterious
Well, you said that as physics gets more advanced it becomes more difficult to understand intuitively. I disagree because I think a lot of things are far better understood when taken beyond the scope of A-level teaching. So things like nuclear fission energy, and a lot of other tripe that they bung into the A-level syllabus, are too lightly skimmed over for it to be really understood. They're trying to make it easier, but it seems to be that it's only 'easier' if you simply memorise the right bits to pass the exams. Hence people like the OP asking for more info, because the way it's presented at sixth form is far too watered down for it to make any sense at all.
At undergrad level I think stuff gets more intuitive because you gain a much deeper grasp on the real workings of all the processes. Honestly I can't believe how much of a bodge the current general physics A-level syllabus is. So much info with no explanation. Hell, I don't even know why they bother teaching physics to students who aren't taking maths as well. Something to do with 'accessibility', I guess. Sorry, bit of a rant, not aimed at you, heh.
In general I just disagree that physics gets less intuitive at more advanced stages. I find it far easier now than I did at A-level.
Anyway.
May or may not be helpful but this is as much as I can add:

Fission chain reaction. The U235 breaks into two smaller atoms. The force/energy holding the smaller atoms together is less than the force/energy that held together the U235. The energy must still exist, it can't just become nothing. So it is emitted and dissipated in it's final form - heat.

At undergrad level I think stuff gets more intuitive because you gain a much deeper grasp on the real workings of all the processes. Honestly I can't believe how much of a bodge the current general physics A-level syllabus is. So much info with no explanation. Hell, I don't even know why they bother teaching physics to students who aren't taking maths as well. Something to do with 'accessibility', I guess. Sorry, bit of a rant, not aimed at you, heh.
In general I just disagree that physics gets less intuitive at more advanced stages. I find it far easier now than I did at A-level.
Anyway.
May or may not be helpful but this is as much as I can add:

Fission chain reaction. The U235 breaks into two smaller atoms. The force/energy holding the smaller atoms together is less than the force/energy that held together the U235. The energy must still exist, it can't just become nothing. So it is emitted and dissipated in it's final form - heat.

So, the potential energy lost (that little tug inwards by the strong force) is equal to mc^2 and also equal to binding energy per nucleon, which is why that graph is important.
thats my little a level model that I'm running past you guys. Again, I apologise for my incoherence.
(edited 10 years ago)
Original post by genuinelydense
thanks for the (long!) reply. I'm pretty sure I e=mc^2 at 'advanced' level: if mass is lost, energy is lost - converted into KE/light? the picture I've built up in my mind is that due to the ranges of the electrostatic and strong nuclear forces, very large nuclei have weaker bonds (I'm using the analogy of bond lengths from chemistry). When they split up, the nucleons are brought a tiny bit closer together (work is done by the strong nuclear force and hence potential of the nucleons decreases). Then, via some obviously non-Newtonian mechanism (I'm imagining 2 spherical balls being brought a little closer together, in which case they would essentially 'hit' each other head on, resulting in a roughly elastic collision than would not increase their combined velocity at all) they lose mass and begin to move really fast.
So, the potential energy lost (that little tug inwards by the strong force) is equal to mc^2 and also equal to binding energy per nucleon, which is why that graph is important.
thats my little a level model that I'm running past you guys. Again, I apologise for my incoherence.
So, the potential energy lost (that little tug inwards by the strong force) is equal to mc^2 and also equal to binding energy per nucleon, which is why that graph is important.
thats my little a level model that I'm running past you guys. Again, I apologise for my incoherence.
Hmm, I think the main thing worth noting for now is the simple explanation:
Hydrogen fusion is this -
H + H >>> He
This seems sort of reasonable. But it doesn't explain much.
By breaking this down further you can see it from the constituent parts of the atoms:
(p+p) + (p+p) >>> (p+p+n+n)
As you can see, somewhere in that mess of a reaction, two of the protons have become neutrons.
Neutrons are lighter than protons. So mass is lost in the process.
In finer detail:
Einstein didn't actually derive mc^2 and shout, "Eureka! All mass is energy!". E=mc^2 was just a by-product of his work on understanding the nature of objects as they travel at velocities approaching the speed of light.
Spoiler
E=mc^2 was hard to verify until it was seen that pions decayed into a pure electromagnetic wave, with energy that measured exactly [(mass of pion)*c^2 ]. Hence they saw that the entire pion had become pure energy.
The pion example is proof that subatomic particles exist as nothing more than a state of perfectly bundled energy (sorry I can't explain it better than that - can anyone?).
Hence in a nuclear reaction, the same kind of thing happens. Mass is lost as energy, just as the mass of the pion was lost.
Quick Reply
Related discussions
- Binding Energy (Isaac Physics J4.8 Part A)
- Chemistry relative atomic mass
- A level revision songs
- AQA A Level Physics Paper 2 7408/2 - 10 Jun 2022 [Exam Chat]
- 2018 OCR A level exploring physics - question 25a)ii)
- Edexcel A Level Physics Advanced Physics II 9PH0 02 - 9th June 2023 [Exam Chat]
- How do I know whether its my body image perception or if someone is actually fat?
- Alevel bio synoptic essay
- Chemistry heating curve question
- If anorexia has an effect on the mind, why did I not struggle academically in school?
- Physics mass-energy question.
- Physics MCQ
- PAT space question
- Physics ice melting SHC and latent heat question
- A level physics question on gravitational fields
- Specific Heat Capacity help G4.2 (a+b)
- Biology question (OCR A 5.1.2 excretion)
- TOF Mass Spec
- Hard Enthalpy Change Question - Help Needed
- Wind Turbine Power




