Entropy type of question
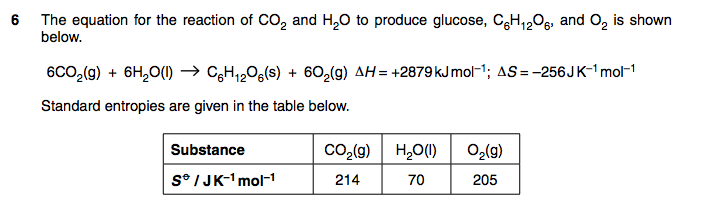
Any method for this? Haven't come across this type of question before.
Original post by TheNoobishKnight
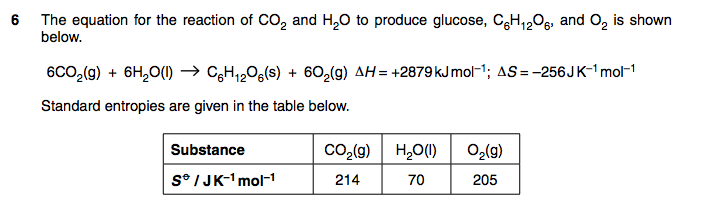
Any method for this? Haven't come across this type of question before.

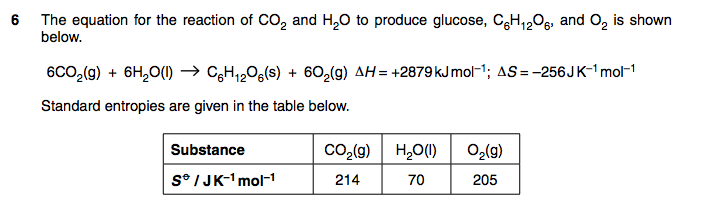
Any method for this? Haven't come across this type of question before.
Entropy is an absolute quantity. It is also extensive.
Hence the entropy change is equal to the entropy of the products - the entropy of the reactants.
Original post by charco
Entropy is an absolute quantity. It is also extensive.
Hence the entropy change is equal to the entropy of the products - the entropy of the reactants.
Hence the entropy change is equal to the entropy of the products - the entropy of the reactants.
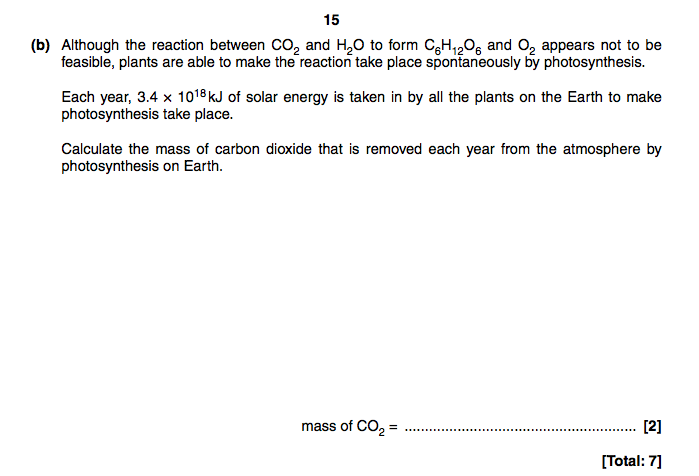
When I looked on the mark scheme, first you had to divide that big number by the delta H. Then you had to do something else.
But I don't understand what you are telling here? Do you mean SP-SR?
Original post by TheNoobishKnight
When I looked on the mark scheme, first you had to divide that big number by the delta H. Then you had to do something else.
But I don't understand what you are telling here? Do you mean SP-SR?
But I don't understand what you are telling here? Do you mean SP-SR?
You divide by delta H to find the number of moles of glucose produced from that much energy, since enthalpy is in kJ/mol. You'd then want to multiply by 6 to give the number of moles of CO2. Since from the balanced equation there is 6:1 ratio of CO2:glucose. Then use the molar mass of CO2 to work out the mass taken in by the plants
Original post by TheNoobishKnight
When I looked on the mark scheme, first you had to divide that big number by the delta H. Then you had to do something else.
But I don't understand what you are telling here? Do you mean SP-SR?
But I don't understand what you are telling here? Do you mean SP-SR?
Maybe it's just me but I can't actually see a question in the part about entropy ...

Original post by charco
Maybe it's just me but I can't actually see a question in the part about entropy ... 

Sorry I got it now thanks to the above user.
I posted that part as it was required to work out the question.
Quick Reply
Related discussions
- Question on entropy changes
- Thermodynamics question
- Equilibria and entropy changes at boiling temperature
- AQA A Level Chemistry Thermodynamics
- A level chem Mc question help
- Calculate the gibbs energy, entropy, and enthalpy of mixing when 1.00 mol C6H14
- Chemistry - transition metals
- a level isaac chemistry question
- Any tips on revising for A-Levels in Y12
- Personal statement help for biological natural sciences
- epq physics question
- Edexcel A-Level Chem Paper 1 Advanced Inorganic and Physical Chemistry [Exam Chat]
- A level Chemistry Uplearn
- Chemistry - solubility and enthalpy of hydration
- Edexcel A-Level Chemistry Paper 1 Core Inorganic and Physical Chemistry [Exam Chat]
- Road to Gold: Physics Olympiads
- Understanding exam questions
- bio help a level
- Natural sciences
- IAL Chemistry - Unit 4 EXAM DISCUSSION
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



