AS Chemistry- helping each other out!
Scroll to see replies
Original post by zhang-liao
University of London Taster Courses
http://www.london.ac.uk/TASTERS
http://www.london.ac.uk/TASTERS
Thanks! It looks really good but I can't find any info on stem ones?
Posted from TSR Mobile
Original post by noshahmad
Lol please. I haven't even begun revising yet. I hope to start tomorrow I've been putting it off.
I'm excited for year 13, I can finally drop Economics which I detest but I don't want to find next year really difficult, its reassuring its not too bad though
Posted from TSR Mobile
I'm excited for year 13, I can finally drop Economics which I detest but I don't want to find next year really difficult, its reassuring its not too bad though

Posted from TSR Mobile
haha, I have done a good few hours, these days averaging 4-5 hours, hopefully keeping the flow going throughout the year and upping the wattage when it gets close to the exam.
It won't be hard if you put the effort it, that's pretty much it.
Also make sure you use the right books, for the courses. I used, for psychology, the CGP which really wasn't all that good and managed to get a B, but maybe if I used the official one with a lot more info, It would have gotten me a better grade( and the fact that I should have revised more!).
Just keep that in mind, cause you don't want to end up skipping important stuff!
Original post by noshahmad
I hope to apply for Chemistry, although I wouldn't mind biology either so I'll try and accomadate both in my PS
Posted from TSR Mobile
I hope to apply for Chemistry, although I wouldn't mind biology either so I'll try and accomadate both in my PS

Posted from TSR Mobile
Man I forgot I have to gear it towards several subjects -_- mty back ups are biology and physics and maths so I'm not sure how this will work out
 I just equally enjoy each
I just equally enjoy each 
If we're both doing one with chemistry in mind we could probably help each other out

Posted from TSR Mobile
Original post by TheNoobishKnight
haha, I have done a good few hours, these days averaging 4-5 hours, hopefully keeping the flow going throughout the year and upping the wattage when it gets close to the exam.
It won't be hard if you put the effort it, that's pretty much it.
Also make sure you use the right books, for the courses. I used, for psychology, the CGP which really wasn't all that good and managed to get a B, but maybe if I used the official one with a lot more info, It would have gotten me a better grade( and the fact that I should have revised more!).
Just keep that in mind, cause you don't want to end up skipping important stuff!
It won't be hard if you put the effort it, that's pretty much it.
Also make sure you use the right books, for the courses. I used, for psychology, the CGP which really wasn't all that good and managed to get a B, but maybe if I used the official one with a lot more info, It would have gotten me a better grade( and the fact that I should have revised more!).
Just keep that in mind, cause you don't want to end up skipping important stuff!
Yeah I've bought the revision guides for all my subjects and they help so much. I use them more than the textbook but refer to it in case I missed a point but I cross check with my spec.
Yeah hopefully I'll average in 5 hours until school open. Ideally want to have learnt the course by Feb half term so I can spend the rest of the time revising/doing past papers but I know thats a real stretch, good luck!

Posted from TSR Mobile
Original post by lyricalvibe
Man I forgot I have to gear it towards several subjects -_- mty back ups are biology and physics and maths so I'm not sure how this will work out  I just equally enjoy each
I just equally enjoy each 
If we're both doing one with chemistry in mind we could probably help each other out
Posted from TSR Mobile
 I just equally enjoy each
I just equally enjoy each 
If we're both doing one with chemistry in mind we could probably help each other out

Posted from TSR Mobile
Doing all 4 will be difficult. I guess they do all compliment each other so you can talk about it vaguely but still having some sort of focus. I just checked and it said the deadline is in March for those in London?
Posted from TSR Mobile
Original post by lyricalvibe
Sorry bout the late reply, went to sleep

They open all their taster courses on the 11th of January I believe
Original post by HAnwar
Sorry if this question's already been asked. Does anyone know the dates of the Chemistry exam, OCR A?
First one's on Atoms Bonds and Groups on the 22nd May, Second- Chains, Energy and Resources on the 2nd June

I'm doing aqa! Such a good idea
Original post by Dinaa
Hello AS Chemistry TSRians!
I thought it would be a good idea to make a thread, where students doing AS Chemistry, post/ask questions, and we could all help each other out?
Teaching another person, also helps you become more confident and you both develop a better understanding. I know there is an A-level chat thread already, but this should be for learning!
There is no such thing as a silly question, so ask away and hopefully, you'll find someone who can help you
I shall also make a list of the Chemistry students, and what exam board they're all on! So please post what exam board you are on, if you are in!
List of the students doing Chemistry!
Lovely helpers!
I apologize if I've missed anyone out, got the list from Current year 12 list! (Lazy i know)
P.S- If you don't want to, okay, you are not obliged to do so! But please, no rude/offensive comments










I thought it would be a good idea to make a thread, where students doing AS Chemistry, post/ask questions, and we could all help each other out?
Teaching another person, also helps you become more confident and you both develop a better understanding. I know there is an A-level chat thread already, but this should be for learning!
There is no such thing as a silly question, so ask away and hopefully, you'll find someone who can help you

I shall also make a list of the Chemistry students, and what exam board they're all on! So please post what exam board you are on, if you are in!
List of the students doing Chemistry!
Spoiler
Lovely helpers!
Spoiler
I apologize if I've missed anyone out, got the list from Current year 12 list! (Lazy i know)

P.S- If you don't want to, okay, you are not obliged to do so! But please, no rude/offensive comments











Hiiii, can you please add me as well?
 I'm on Edexcel
I'm on Edexcel 
Sorry if this has been asked but it's a question I have been struggling on for a while. I think I know how to do it as I have tried to self teach but I'm wondering what methods other people use to see if they make more sense.
How do you work out % yield?
How do you work out % yield?
Original post by Charlblackburn
Sorry if this has been asked but it's a question I have been struggling on for a while. I think I know how to do it as I have tried to self teach but I'm wondering what methods other people use to see if they make more sense.
How do you work out % yield?
How do you work out % yield?
(Actual Yield/Theoretical Yield) x 100% = % yield
Edexcel provide the actual yield however sometimes expect you to calculate the theoretical yield, don't know about OCR or AQA
To calculate the theoretical yield you can calculate the mass calculated using molar calculations in the reaction in the question I believe (e.g no. of moles x Mr = mass, etc.)
(edited 9 years ago)
Original post by PepperPotts
First one's on Atoms Bonds and Groups on the 22nd May, Second- Chains, Energy and Resources on the 2nd June 

Thanks!
Original post by GoldGhost
What's the difference between Cis, trans and E/Z isomerism? I don't understand the explanation in the textbook.
Im OCR A if that helps! :-)
Im OCR A if that helps! :-)
Im doing AQA, so this may explain too much/not enough but here goes.
Firstly, I remember it as E/Z, not Cis/Trans as E/Z tells me what the isomers look like.
I'm assuming that you know that each of the Carbon atoms must have two different groups attached to them and that the stereoisomers "have the same structural formula, just a different arrangement in space" (the "...." is a 1-2 mark answer which I know off by heart, as it is often asked).
Here are two stereoisomers:
I remember the E/Z from what they look like; the one that looks like an 'E', is the Z (Draw a Z in midair across the stereoisomer: For the first it would go from H-Cl-Cl-H and the 2nd does not look like a Z.)
Edit: The trans is the one that has same groups diagonally across from each other.
This shows a way to explain which is E and Z and which is Cis and Trans, but I believe that you only need to know one of them.
If you need to know anything else, just say.
The drawing E/Z in midair may be confusing from my writing as it is hard to explain without doing it in front of you.
Original post by l1lvink
Im doing AQA, so this may explain too much/not enough but here goes.
Firstly, I remember it as E/Z, not Cis/Trans as E/Z tells me what the isomers look like.
I'm assuming that you know that each of the Carbon atoms must have two different groups attached to them and that the stereoisomers "have the same structural formula, just a different arrangement in space" (the "...." is a 1-2 mark answer which I know off by heart, as it is often asked).
Here are two stereoisomers:
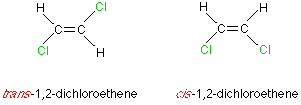
I remember the E/Z from what they look like; the one that looks like an 'E', is the Z (Draw a Z in midair across the stereoisomer: For the first it would go from H-Cl-Cl-H and the 2nd does not look like a Z.)
Edit: The trans is the one that has same groups diagonally across from each other.
This shows a way to explain which is E and Z and which is Cis and Trans, but I believe that you only need to know one of them.
If you need to know anything else, just say.
The drawing E/Z in midair may be confusing from my writing as it is hard to explain without doing it in front of you.
Firstly, I remember it as E/Z, not Cis/Trans as E/Z tells me what the isomers look like.
I'm assuming that you know that each of the Carbon atoms must have two different groups attached to them and that the stereoisomers "have the same structural formula, just a different arrangement in space" (the "...." is a 1-2 mark answer which I know off by heart, as it is often asked).
Here are two stereoisomers:
I remember the E/Z from what they look like; the one that looks like an 'E', is the Z (Draw a Z in midair across the stereoisomer: For the first it would go from H-Cl-Cl-H and the 2nd does not look like a Z.)
Edit: The trans is the one that has same groups diagonally across from each other.
This shows a way to explain which is E and Z and which is Cis and Trans, but I believe that you only need to know one of them.
If you need to know anything else, just say.
The drawing E/Z in midair may be confusing from my writing as it is hard to explain without doing it in front of you.
Another easy way to learn E/Z rules is that E = epposite side (I know its cheesy) and Z = zame zide

Original post by zhang-liao
Another easy way to learn E/Z rules is that E = epposite side (I know its cheesy) and Z = zame zide 

Hmm, I like the zame zide.
I wonder how many other ways there are of remembering this.
Original post by l1lvink
Hmm, I like the zame zide.
I wonder how many other ways there are of remembering this.
I wonder how many other ways there are of remembering this.
Courtesy of Chemrevise

Original post by GoldGhost
What's the difference between Cis, trans and E/Z isomerism? I don't understand the explanation in the textbook.
Im OCR A if that helps! :-)
Im OCR A if that helps! :-)
E/Z is a more complete way of designating stereoisomers as it can be used for unsaturated compounds that don't have two groups the same. eg FClC=CBrI
Cis-trans description requires that at least two of the groups attached to the double bond are identical.
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- Chemistry Degree Lab Work
- Making chemistry notes
- Physical natural sciences
- !!! A level choices - Chem or Computer science
- A level chemistry for a biology degree? (help please!!)
- Do I have enough time to turn my grades around??
- Mass vs relative atomic mass on periodic table
- Official NATURAL SCIENCES applicants thread 2024
- What is it like doing chem vs natural sciences at uni?
- in need of urgent advice, would appreciate any help :)
- Study leave
- Lancaster uni timetable
- Need help on a Venn diagram question
- Biochemistry at University
- Higher chemistry
- Cambridge Natural Sciences Interview Applicants 2023/24
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me?
- Save me from a level torture
Latest
Last reply 1 minute ago
Is it really such a bad thing to have an undefined relationshipLast reply 4 minutes ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 5 minutes ago
Best SEO Service Company In USALast reply 8 minutes ago
Worried about the acceptance of my A level Qualifications. Need an answerLast reply 9 minutes ago
Name a country or city with the last letter of the previous oneForum games
7298
Last reply 10 minutes ago
Amazon Project management apprenticeship 2024Last reply 22 minutes ago
SQA Higher Physics - Paper 2 - 25th April 2024 [Exam Chat]Last reply 25 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2928
Last reply 27 minutes ago
Apprenticeship needs a 4 in scienceTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 5 days ago
Im confused about this chemistry question, why does it form these products



