Electrochemical cell emf question
(This is from A2 Chemistry but I think the question is more physical than chemical)
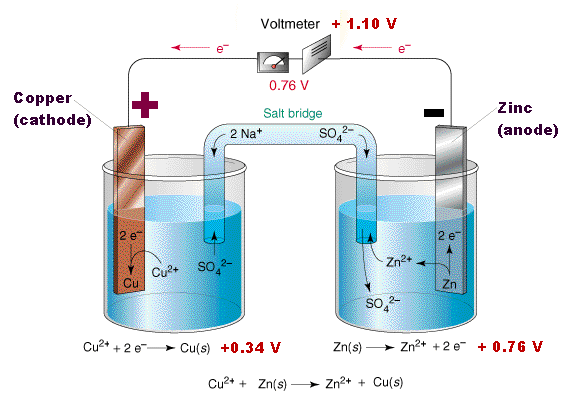
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
Dont know if this helps but my revision guides says this 'diluting the solution reduces the concentration of the metal ion and thus disturbs the equilibrium, which shifts to the left to compensate, forming more electrons and therefore making the half cell electrode potential more negative '
EMF is positive volts, potential difference is negative volts
Original post by Audiology-Med
Dont know if this helps but my revision guides says this 'diluting the solution reduces the concentration of the metal ion and thus disturbs the equilibrium, which shifts to the left to compensate, forming more electrons and therefore making the half cell electrode potential more negative '
I completely understand that, but I don't see why more electrons = greater EMF since EMF is J/c?
Original post by shuheb789
EMF is positive volts, potential difference is negative volts
I don't see how that answers my question?

what exam paper is this question on by the way
Original post by Chlorophile
I completely understand that, but I don't see why more electrons = greater EMF since EMF is J/c?
I don't see how that answers my question?
I don't see how that answers my question?

Dont know if you worked it out or not but I thin this explains it more, I take no credit for the following explanation, this is what someone else said;
"If the concentration of Fe3+ increases, then the emf for that half cell will decrease as when written as a reduction potential Fe3+ + e- -> Fe2+ + 0.77V, an increase in Fe3+ concentration will cause an accumulation of negative charge as more electrons are lost. The emf of the cell is calculated by E = E(red) - E(ox) so as the emf of iron becomes more negative, the overall emf increases , sorry that's probably badly explained but it makes sense to me lol"
I think we kind of explains it well
In simple terms, e.m.f. is a function of the difference in atomic shell energy levels between the donor and receptor atoms.
In order for electrons to transfer between electrodes an energy potential must exist. i.e. the redox reactions involved must have a potential for the anode to become oxidized and the cathode to become reduced.
Electrons will 'fall' from the higher potential (to become oxidised) anode to the lower potential (to become reduced) cathode. The difference between the anode's potential and the cathode's potential is the cell potential.
The cell potential is the difference between the two electrodes:
where is the standard cell potential.
The standard cell e.m.f. potential will be determined by the electron donor and electron acceptor atoms.
i.e. the free energy in the reduced form relative to the free energy in the oxidised form which are dependant on the positions within the atomic shells of the atoms from whence they are donated or accepted.
Which goes back to the reactivity series of the periodic table. i.e. the difference in shell energy levels of the occupying electrons donated by the parent atom to that of the vacant shell energy levels occupied by the transferred electrons at the receptor atom.
And since shell energy levels are a quantum property, the e.m.f. of a given cell is fixed by it's constituent elements.
(For further info, look up Nernst Equation).
In order for electrons to transfer between electrodes an energy potential must exist. i.e. the redox reactions involved must have a potential for the anode to become oxidized and the cathode to become reduced.
Electrons will 'fall' from the higher potential (to become oxidised) anode to the lower potential (to become reduced) cathode. The difference between the anode's potential and the cathode's potential is the cell potential.
The cell potential is the difference between the two electrodes:
where is the standard cell potential.
The standard cell e.m.f. potential will be determined by the electron donor and electron acceptor atoms.
i.e. the free energy in the reduced form relative to the free energy in the oxidised form which are dependant on the positions within the atomic shells of the atoms from whence they are donated or accepted.
Which goes back to the reactivity series of the periodic table. i.e. the difference in shell energy levels of the occupying electrons donated by the parent atom to that of the vacant shell energy levels occupied by the transferred electrons at the receptor atom.
And since shell energy levels are a quantum property, the e.m.f. of a given cell is fixed by it's constituent elements.
(For further info, look up Nernst Equation).
(edited 9 years ago)
Original post by uberteknik
In simple terms, e.m.f. is a function of the difference in atomic shell energy levels between the donor and receptor atoms.
In order for electrons to transfer between electrodes an energy potential must exist. i.e. the redox reactions involved must have a potential for the anode to become oxidized and the cathode to become reduced.
Electrons will 'fall' from the higher potential (to become oxidised) anode to the lower potential (to become reduced) cathode. The difference between the anode's potential and the cathode's potential is the cell potential.
The cell potential is the difference between the two electrodes:
where is the standard cell potential.
The standard cell e.m.f. potential will be determined by the electron donor and electron acceptor atoms.
i.e. the free energy in the reduced form relative to the free energy in the oxidised form which are dependant on the positions within the atomic shells of the atoms from whence they are donated or accepted.
Which goes back to the reactivity series of the periodic table. i.e. the difference in shell energy levels of the occupying electrons donated by the parent atom to that of the vacant shell energy levels occupied by the transferred electrons at the receptor atom.
And since shell energy levels are a quantum property, the e.m.f. of a given cell is fixed by it's constituent elements.
(For further info, look up Nernst Equation).
In order for electrons to transfer between electrodes an energy potential must exist. i.e. the redox reactions involved must have a potential for the anode to become oxidized and the cathode to become reduced.
Electrons will 'fall' from the higher potential (to become oxidised) anode to the lower potential (to become reduced) cathode. The difference between the anode's potential and the cathode's potential is the cell potential.
The cell potential is the difference between the two electrodes:
where is the standard cell potential.
The standard cell e.m.f. potential will be determined by the electron donor and electron acceptor atoms.
i.e. the free energy in the reduced form relative to the free energy in the oxidised form which are dependant on the positions within the atomic shells of the atoms from whence they are donated or accepted.
Which goes back to the reactivity series of the periodic table. i.e. the difference in shell energy levels of the occupying electrons donated by the parent atom to that of the vacant shell energy levels occupied by the transferred electrons at the receptor atom.
And since shell energy levels are a quantum property, the e.m.f. of a given cell is fixed by it's constituent elements.
(For further info, look up Nernst Equation).
That's exactly what I thought, so does that mean the question is wrong, i.e. the concentration wouldn't affect the e.m.f? Or am I completely misunderstanding you?
Original post by Chlorophile
That's exactly what I thought, so does that mean the question is wrong, i.e. the concentration wouldn't affect the e.m.f? Or am I completely misunderstanding you?
The question is OK. I should have added 'the maximum e.m.f. of the cell is fixed by it's constituent elemnets'.
Don't forget that voltage is nothing more than a measure of the potential to do work.
The chemical energy available in the system is still a function of the concentrations of the aqueous solution.
The more +ve the potential, the more likely it will be reduced.
Rather like a brick raised above ground and sitting on a ledge has potential energy. Raising the brick or lowering it changes the bricks potential. The gravitational constant does not change.
The e.m.f. of the cell is therefore a statement of the chemical potential still available to be liberated for that redox reaction at any given time.
As the redox reaction commences, the potential energy of the system immediately begins to fall and since e.m.f. is a measure of potential, it too must fall until the reaction is complete when the e.m.f. will be zero.
(edited 9 years ago)
Original post by uberteknik
The question is OK. I should have added 'the maximum e.m.f. of the cell is fixed by it's constituent elemnets'.
Don't forget that voltage is nothing more than a measure of the potential to do work.
The chemical energy available in the system is still a function of the concentrations of the aqueous solution.
The more +ve the potential, the more likely it will be reduced.
Rather like a brick raised above ground and sitting on a ledge has potential energy. Raising the brick or lowering it changes the bricks potential. The gravitational constant does not change.
The e.m.f. of the cell is therefore a statement of the chemical potential still available to be liberated for that redox reaction at any given time.
As the redox reaction commences, the potential energy of the system immediately begins to fall and since e.m.f. is a measure of potential, it too must fall until the reaction is complete when the e.m.f. will be zero.
Don't forget that voltage is nothing more than a measure of the potential to do work.
The chemical energy available in the system is still a function of the concentrations of the aqueous solution.
The more +ve the potential, the more likely it will be reduced.
Rather like a brick raised above ground and sitting on a ledge has potential energy. Raising the brick or lowering it changes the bricks potential. The gravitational constant does not change.
The e.m.f. of the cell is therefore a statement of the chemical potential still available to be liberated for that redox reaction at any given time.
As the redox reaction commences, the potential energy of the system immediately begins to fall and since e.m.f. is a measure of potential, it too must fall until the reaction is complete when the e.m.f. will be zero.
Still, I'm a bit confused. You said that the energy changes are discrete and depends on the element. So whilst I completely understand that increasing the negativity of a terminal increases the likelihood of the substance being oxidised, why does this actually increase the potential per unit of charge rather than simply increasing the rate of flow of charge?
(edited 9 years ago)
Original post by Chlorophile
Still, I'm a bit confused. You said that the energy changes are discrete and depends on the element. So whilst I completely understand that increasing the negativity of a terminal increases the likelihood of the substance being oxidised, why does this actually increase the potential per unit of charge rather than simply increasing the rate of flow of charge?
Let's put it another way the charge displacement building at the electrodes creates an electric field which when large enough then arrests any further reaction.
Charge displacement is a function of the aqueous solution concentration. More charge carriers per mol solution creates a larger electric field density.
Original post by uberteknik
OK.
Let's put it another way the charge displacement building at the electrodes creates an electric field which when large enough then arrests any further reaction.
Charge displacement is a function of the aqueous solution concentration. More charge carriers per mol solution creates a larger electric field density.
Let's put it another way the charge displacement building at the electrodes creates an electric field which when large enough then arrests any further reaction.
Charge displacement is a function of the aqueous solution concentration. More charge carriers per mol solution creates a larger electric field density.
So each actual charge carrier has a higher potential with a high concentration solution than in a low concentration solution?
Original post by Chlorophile
So each actual charge carrier has a higher potential with a high concentration solution than in a low concentration solution?
You are nearly there. Let's put all the previous posts together!
Go back a bit. When a metal electrode is placed within an electrolyte, some of the atoms in the electrolyte go into solution as ions while the electrons create a negative charge on the electrode.
The charge on the electrode therefore increases, but it cannot do this indefinitely because the charge build up at the electrode creates an electric field which makes it increasingly difficult for positive metal ions to go into solution.
The separated charge build up therefore creates an e.m.f. at the electrode and (the equilibrium at which the charge arrests a further increase), is a function of the electrolyte concentration. i.e. a greater density of positive ions in solution transferred from the electrode is made possible by reducing the original concentration of Fe3+(aq|) electrolyte to allow more electrode ions to enter solution and increase the charge build up on the electrode before the migration is halted by the build in the electric field at the electrode.
So the voltage pressure created at the electrode is a function of the atomic structure of the electrode. i.e. reactivity and atom density whilst the build up of voltage pressure for any given electrode composition is arrested when charge separation equilibrium is reached with the ionic concentration of the electrolyte.
When the conduction path between the electrode pairs in a battery is completed, electrons will flow between the electrodes via the completed circuit. This immediately reduces the charge at the cathode which allows more ions to go into solution.
Hence the completed circuit allows the reaction to continue at a rate governed by the charge separation equilibrium point at the electrodes and the resistance of the conduction path between the electrodes.
It's rather analogous to the energy stored in the electric field between the plates of a capacitor, but this time it's the electric field created between the electrode and the electrolyte and migration of electrons allows the redox reactions to continue until the electrolyte is depleted.
Hence battery capacity is a function of the electrolyte volume.
E.M.F. is a function of the electrode atomic shell configuration and original concentration of ions in the electrolyte solution.
(edited 9 years ago)
Original post by Audiology-Med
Dont know if you worked it out or not but I thin this explains it more, I take no credit for the following explanation, this is what someone else said;
"If the concentration of Fe3+ increases, then the emf for that half cell will decrease as when written as a reduction potential Fe3+ + e- -> Fe2+ + 0.77V, an increase in Fe3+ concentration will cause an accumulation of negative charge as more electrons are lost. The emf of the cell is calculated by E = E(red) - E(ox) so as the emf of iron becomes more negative, the overall emf increases , sorry that's probably badly explained but it makes sense to me lol"
I think we kind of explains it well
"If the concentration of Fe3+ increases, then the emf for that half cell will decrease as when written as a reduction potential Fe3+ + e- -> Fe2+ + 0.77V, an increase in Fe3+ concentration will cause an accumulation of negative charge as more electrons are lost. The emf of the cell is calculated by E = E(red) - E(ox) so as the emf of iron becomes more negative, the overall emf increases , sorry that's probably badly explained but it makes sense to me lol"
I think we kind of explains it well
Original post by Chlorophile
(This is from A2 Chemistry but I think the question is more physical than chemical)
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
Uberteknik is talking way above what you need to know, the correct answer is above.
Its quite simple, it's just to do with the EMF half equations.
Original post by Schrödingers Cat
Uberteknik is talking way above what you need to know, the correct answer is above.
Its quite simple, it's just to do with the EMF half equations.
Its quite simple, it's just to do with the EMF half equations.
Original post by uberteknik
The OP already knows that. He wanted a more detailed understanding of why the transfer of energy is limited to a finite joules per coulomb of charge and why the concentration of ions in solution affects the e.m.f. of the cell.
It doesn't sound like he does, he doesn't know why the EMF changes which is the fundamental knowledge for understanding in more detail of the question
(edited 9 years ago)
Original post by uberteknik
No.
You are nearly there. Let's put all the previous posts together!
Go back a bit. When a metal electrode is placed within an electrolyte, some of the atoms in the electrolyte go into solution as ions while the electrons create a negative charge on the electrode.
The charge on the electrode therefore increases, but it cannot do this indefinitely because the charge build up at the electrode creates an electric field which makes it increasingly difficult for positive metal ions to go into solution.
The separated charge build up therefore creates an e.m.f. at the electrode and the equilibrium at which the charge arrests a further increase is a function of the electrolyte concentration. i.e. a greater density of positive ions in solution is made possible by reducing the original concentration of Fe3+(aq|) electrolyte to allow more electrode ions to enter solution and increase the charge build up on the electrode before the migration is halted.
So the voltage pressure created at the electrode is a function of the atomic structure of the electrode. i.e. reactivity and atom density whilst the build up of voltage pressure for any given electrode composition is arrested when charge separation equilibrium is reached with the ionic concentration of the electrolyte.
When the conduction path between the electrode pairs in a battery is completed, electrons will flow between the electrodes via the completed circuit. This immediately reduces the charge at the cathode which allows more ions to go into solution.
Hence the completed circuit allows the reaction to continue at a rate governed by the charge separation equilibrium point at the electrodes and the resistance of the conduction path between the electrodes.
It's rather analogous to the energy stored in the electric field between the plates of a capacitor, but this time it's the electric field created between the electrode and the electrolyte and migration of electrons allows the redox reactions to continue until the electrolyte is depleted.
Hence battery capacity is a function of the electrolyte volume.
E.M.F. is a function of the electrode atomic shell configuration and original concentration of ions in the electrolyte solution.

You are nearly there. Let's put all the previous posts together!
Go back a bit. When a metal electrode is placed within an electrolyte, some of the atoms in the electrolyte go into solution as ions while the electrons create a negative charge on the electrode.
The charge on the electrode therefore increases, but it cannot do this indefinitely because the charge build up at the electrode creates an electric field which makes it increasingly difficult for positive metal ions to go into solution.
The separated charge build up therefore creates an e.m.f. at the electrode and the equilibrium at which the charge arrests a further increase is a function of the electrolyte concentration. i.e. a greater density of positive ions in solution is made possible by reducing the original concentration of Fe3+(aq|) electrolyte to allow more electrode ions to enter solution and increase the charge build up on the electrode before the migration is halted.
So the voltage pressure created at the electrode is a function of the atomic structure of the electrode. i.e. reactivity and atom density whilst the build up of voltage pressure for any given electrode composition is arrested when charge separation equilibrium is reached with the ionic concentration of the electrolyte.
When the conduction path between the electrode pairs in a battery is completed, electrons will flow between the electrodes via the completed circuit. This immediately reduces the charge at the cathode which allows more ions to go into solution.
Hence the completed circuit allows the reaction to continue at a rate governed by the charge separation equilibrium point at the electrodes and the resistance of the conduction path between the electrodes.
It's rather analogous to the energy stored in the electric field between the plates of a capacitor, but this time it's the electric field created between the electrode and the electrolyte and migration of electrons allows the redox reactions to continue until the electrolyte is depleted.
Hence battery capacity is a function of the electrolyte volume.
E.M.F. is a function of the electrode atomic shell configuration and original concentration of ions in the electrolyte solution.

Thank you so much for your patience! I think I am starting to get it now. If I understand this correctly, the concentration affects the ability of charge to build up on each electrode. When it is easier for charge to build up, the charge difference and hence difference in potential between the two electrodes is greater, so more work is done when a coulomb of charge moves between the two electrodes. The capacitance analogy makes a lot of sense - increasing the charge difference across the two electrodes increases the potential difference. Am I right in thinking that capacitance is somehow analogous to the fixed quantum energy changes determined by the materials of the electrodes? So the actual fixed energy changes are the kind of "material property" of the system but the actual thing that determines the potential difference is the ability of charge to accumulate on the electrodes which is determined by the concentrations?
Original post by Schrödingers Cat
Uberteknik is talking way above what you need to know, the correct answer is above.
Its quite simple, it's just to do with the EMF half equations.
Its quite simple, it's just to do with the EMF half equations.
I already know "what I need to know", I can carry out the equations just fine. I'm interested in what's actually going on and Uberteknik is completely answering my question.
Original post by Chlorophile
(This is from A2 Chemistry but I think the question is more physical than chemical)
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
The problem is part of a longer question, but basically there's an electrochemical cell and at the negative electrode, we have the reaction Fe2+(aq) -> Fe3+(aq)+e-. The question asks "Deduce what change in the concentration of Fe3+(aq) would cause an increase in the emf of the cell. Explain your answer."
The answer says you should decrease the concentration of Fe3+(aq) as that would result in the equilibrium shifting to the RHS so the electrode would be more negative so the emf would increase. I understand why the equilibrium would shift to the RHS but I don't see why this would change the emf. Emf is measured in volts, i.e. joules per coulomb. All shifting the equilibrium to the RHS would do is increase the charge, I don't see why it would increase the energy per unit of charge since the energy change when Fe(II) is oxidised is constant. I'm guessing that if you increase the negativity of the negative electrode you're somehow increasing the potential difference across the electrodes but I still don't understand how this increases the emf since the energy change in the reaction is constant and hence the emf should stay constant. Thanks in advance!
Original post by Chlorophile
I already know "what I need to know", I can carry out the equations just fine. I'm interested in what's actually going on and Uberteknik is completely answering my question.
I already know "what I need to know", I can carry out the equations just fine. I'm interested in what's actually going on and Uberteknik is completely answering my question.
Ok then

Original post by Chlorophile
Thank you so much for your patience! I think I am starting to get it now. If I understand this correctly, the concentration affects the ability of charge to build up on each electrode. When it is easier for charge to build up, the charge difference and hence difference in potential between the two electrodes is greater
The electrons will flow from the higher potential to the lower and the direction of electron flow via the conduction path between the electrodes defines cathode and anode nomenclature.
.........so more work is done when a coulomb of charge moves between the two electrodes.
The potential energy stored in the electric fields of each half-cell is expended in overcoming the resistance of the load within the electron conduction path between the electrodes.
i.e. the rate at which the energy gets expended is a function of the resistance and e.m.f. as per ohms law. And since the e.m.f. is a measure of the charge 'pressure' build at the electrodes, more current is able to flow for a given resistance.
The capacitance analogy makes a lot of sense - increasing the charge difference across the two electrodes increases the potential difference. Am I right in thinking that capacitance is somehow analogous to the fixed quantum energy changes determined by the materials of the electrodes?
Electrons always tend to fall to the lowest possible energy state and those energy states are a function of the shell orbitals which are a quantum property. The orbitals therefore determine the chemical reactivity and bonding between atoms.
I used the capacitor example as a way of describing the charge build up at the electrodes which then eventually halts the generation of ions in aqueous solution and vice-versa at the other electrode. i.e. the charge build up between the capacitor plates slows and halts further build up as a result of like charge repulsion.
So the actual fixed energy changes are the kind of "material property" of the system but the actual thing that determines the potential difference is the ability of charge to accumulate on the electrodes which is determined by the concentrations?
Yes, the energy exchange is described by the work function. The charge build up determines the e.m.f.
Original post by uberteknik
Correct.
The electrons will flow from the higher potential to the lower and the direction of electron flow via the conduction path between the electrodes defines cathode and anode nomenclature.
The potential energy stored in the electric fields of each half-cell is expended in overcoming the resistance of the load within the electron conduction path between the electrodes.
i.e. the rate at which the energy gets expended is a function of the resistance and e.m.f. as per ohms law. And since the e.m.f. is a measure of the charge 'pressure' build at the electrodes, more current is able to flow for a given resistance.
Electrons always tend to fall to the lowest possible energy state and those energy states are a function of the shell orbitals which are a quantum property. The orbitals therefore determine the chemical reactivity and bonding between atoms.
I used the capacitor example as a way of describing the charge build up at the electrodes which then eventually halts the generation of ions in aqueous solution and vice-versa at the other electrode. i.e. the charge build up between the capacitor plates slows and halts further build up as a result of like charge repulsion.
Yes, the energy exchange is described by the work function. The charge build up determines the e.m.f.
The electrons will flow from the higher potential to the lower and the direction of electron flow via the conduction path between the electrodes defines cathode and anode nomenclature.
The potential energy stored in the electric fields of each half-cell is expended in overcoming the resistance of the load within the electron conduction path between the electrodes.
i.e. the rate at which the energy gets expended is a function of the resistance and e.m.f. as per ohms law. And since the e.m.f. is a measure of the charge 'pressure' build at the electrodes, more current is able to flow for a given resistance.
Electrons always tend to fall to the lowest possible energy state and those energy states are a function of the shell orbitals which are a quantum property. The orbitals therefore determine the chemical reactivity and bonding between atoms.
I used the capacitor example as a way of describing the charge build up at the electrodes which then eventually halts the generation of ions in aqueous solution and vice-versa at the other electrode. i.e. the charge build up between the capacitor plates slows and halts further build up as a result of like charge repulsion.
Yes, the energy exchange is described by the work function. The charge build up determines the e.m.f.
Okay, excellent. Thank you very much for your help!
Quick Reply
Related discussions
- AQA A Level Chemistry Electrochemistry
- Cell in circuit is reversed
- A level physics aqa help!!!!!!
- Really stuck on this electricity physics question. Please help!
- Physics
- Electricity question help
- pls help with my physics
- chemistry help
- Why does potential difference increase when resistance increases?
- ocr a level chemistry
- Energy dissipated
- Chem a level - electrochemistry
- Circuits Question
- Edexcel A Level Biology B Paper 2: 9BI0 02 - 17 Jun 2022 [Exam Chat]
- Electrcity Help
- OCR A-level chemistry practicals
- Chemistry
- 25 mark essay question
- Electrochemical cells AQA A Level chemistry
- How would an induced emf graph look like if magnetic flux varies in a sine curve?
Latest
Last reply 1 minute ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 minute ago
Accenture Degree Apprenticeship 2024Last reply 2 minutes ago
November 2023 Illegal Migration Intake Unit - Intake Response Team - Immigration OffiLast reply 2 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 3 minutes ago
Medical doctor degree apprenticeship 2024Last reply 4 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadPosted 7 minutes ago
Is MathsFM French Alevels good for a CS degree at top uni (OX, UCL, Durham etc)Last reply 8 minutes ago
Official: Queen's University Belfast A100 2024 Entry ApplicantsLast reply 8 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2843
Last reply 9 minutes ago
Standard Chartered Apprenticeships 2024Last reply 10 minutes ago
Jaguar (JLR) Degree apprenticeships 2024



