AS Chemistry- helping each other out!
Scroll to see replies
Original post by TARS
Type/write notes in your own words. Then do every single past paper.
Make a Microsoft Excel document with all of your exam marks, and have a column for each chapter, where you can add notes for things you do not understand. It really helps.
Like this:
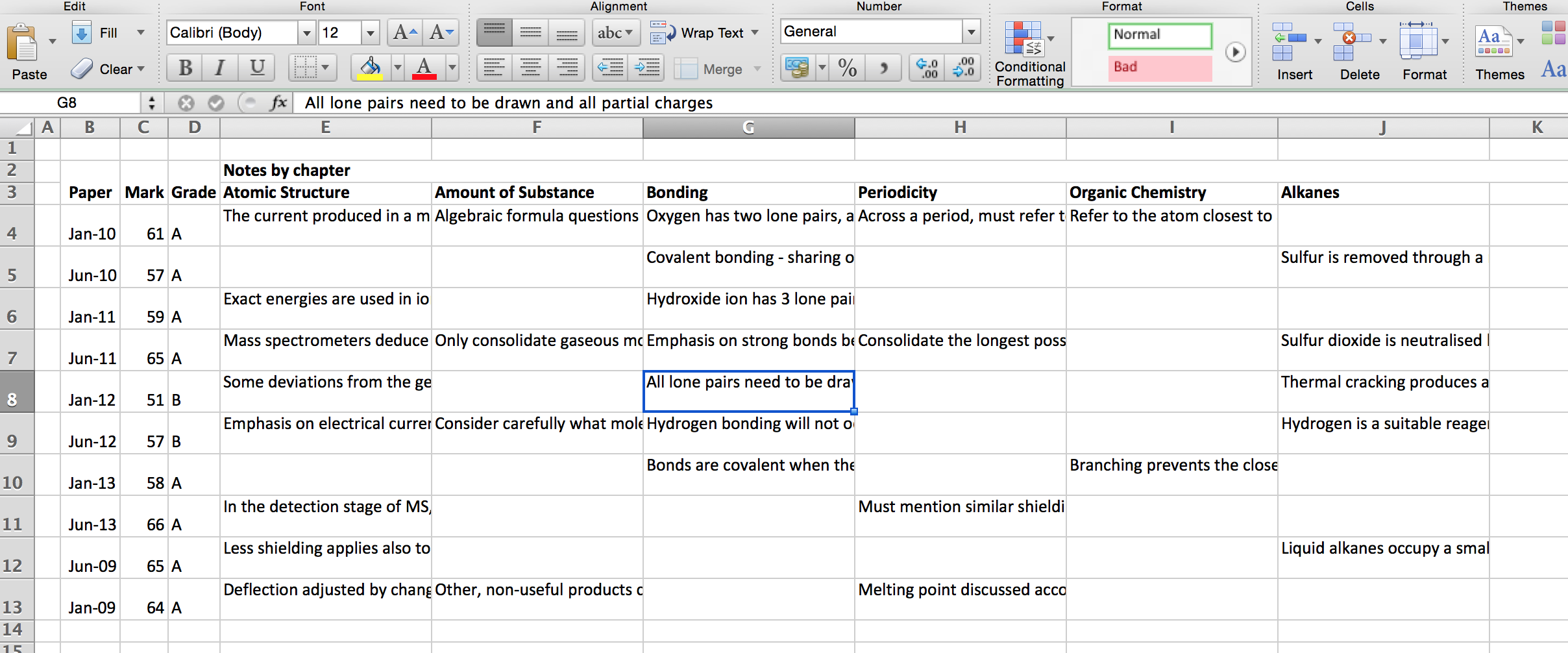
Make a Microsoft Excel document with all of your exam marks, and have a column for each chapter, where you can add notes for things you do not understand. It really helps.
Like this:
Oh my gosh, you're very creative! I may sound naïve but how do you think of these things? You all seem so dedicated and prepared- I don't even have time to do this because I somehow entered a sad position. But I've started studying and 'learning' the content now.
Original post by Cherry82
Oh my gosh, you're very creative! I may sound naïve but how do you think of these things? You all seem so dedicated and prepared- I don't even have time to do this because I somehow entered a sad position. But I've started studying and 'learning' the content now.
It was my physics teacher's idea really, but I just did it and tried it out and it seemed to work so i continued it for all past papers
Original post by Cherry82
Thanks for the link 
But honestly, if someone purely used past papers without learning or reading the content from the book- what grade could they receive as these questions seem almost recycled. Say if I did 20 past papers- could I get maybe grade B through only doing that and looking at mark schemes?

But honestly, if someone purely used past papers without learning or reading the content from the book- what grade could they receive as these questions seem almost recycled. Say if I did 20 past papers- could I get maybe grade B through only doing that and looking at mark schemes?
there's nothing stopping you get 75+ doing that, i'd say there's 5 marks max of new stuff per paper
Original post by BBeyond
there's nothing stopping you get 75+ doing that, i'd say there's 5 marks max of new stuff per paper
Isn't the paper out of 60 though, or is this in the sense of UMS points or something? But really, this sounds too simple though. If it was that easy why isn't everyone doing this? lol- this would be very risky for someone like me who wasn't gone through the whole book and who doesn't know the pieces of the content...
I don't know why but I really hate that official study guide ocr set students. Actually I think it's due to the fact that it doesn't really point out things, there's just large pieces of content without highlighted, underlined info. I had to buy another study guide.
Original post by Cherry82
Isn't the paper out of 60 though, or is this in the sense of UMS points or something? But really, this sounds too simple though. If it was that easy why isn't everyone doing this? lol- this would be very risky for someone like me who wasn't gone through the whole book and who doesn't know the pieces of the content...
I don't know why but I really hate that official study guide ocr set students. Actually I think it's due to the fact that it doesn't really point out things, there's just large pieces of content without highlighted, underlined info. I had to buy another study guide.
I don't know why but I really hate that official study guide ocr set students. Actually I think it's due to the fact that it doesn't really point out things, there's just large pieces of content without highlighted, underlined info. I had to buy another study guide.
Because you still have to learn the answers and its much harder to learn the answers when you don't even understand the concept hence why past papers are usually the kinda thing an A grade student does for full ums rather than a D grade student for a B.also most don't use them correctly and it can be quite time consuming.
For my biology mock I did 1 past paper for practice and it helped for around 20 marks/80, plus 1 of the questions was a exact copy.
Posted from TSR Mobile
Original post by lyricalvibe
Because you still have to learn the answers and its much harder to learn the answers when you don't even understand the concept hence why past papers are usually the kinda thing an A grade student does for full ums rather than a D grade student for a B.also most don't use them correctly and it can be quite time consuming.
For my biology mock I did 1 past paper for practice and it helped for around 20 marks/80, plus 1 of the questions was a exact copy.
Posted from TSR Mobile
For my biology mock I did 1 past paper for practice and it helped for around 20 marks/80, plus 1 of the questions was a exact copy.
Posted from TSR Mobile
Wait, so sorry no offence but what's the point you're making? That past papers do or do not help depending on?
Original post by Cherry82
Wait, so sorry no offence but what's the point you're making? That past papers do or do not help depending on?
Oh sorry, I was replying to the, "if its that easy why doesn't everyone do it"

I personally find them really useful, but I only like using them in conjunction with revision guides.
Posted from TSR Mobile
(edited 8 years ago)
Original post by lyricalvibe
Oh sorry, I was replying to the, "if its that easy why doesn't everyone do it" 
I personally find them really useful, but I only like using them as a supplement to revision guides.
Posted from TSR Mobile

I personally find them really useful, but I only like using them as a supplement to revision guides.
Posted from TSR Mobile
Oh, I understand now. There's been previous articles though on OCR doing this- using old past paper questions like from 2004 in recent papers. I remember reading an article or something- this was like in 2012. How students who did the 2002 past paper got 10 marks free for their A2 exam. Now that it's been raised, I'm sure OCR wouldn't do this again but those students were lucky.
Original post by Cherry82
Oh, I understand now. There's been previous articles though on OCR doing this- using old past paper questions like from 2004 in recent papers. I remember reading an article or something- this was like in 2012. How students who did the 2002 past paper got 10 marks free for their A2 exam. Now that it's been raised, I'm sure OCR wouldn't do this again but those students were lucky.
Are you OCR B? My teachers said its quite likely this'll happen as its the last year for the spec, are you planning on looking at old spec stuff?
Posted from TSR Mobile
Original post by lyricalvibe
Are you OCR B? My teachers said its quite likely this'll happen as its the last year for the spec, are you planning on looking at old spec stuff?
Posted from TSR Mobile
Posted from TSR Mobile
Oh no OCR A. OCR B seems more interesting, wish we did it. Really? They're probably right but who knows.
I was just thinking since it's the last official year for this type of A level- maybe they might do so. I mean we still have resit exams in 2016 specialised for our year only but I doubt that they would want many students to resit the paper next year so maybe the paper this year papers wouldn't be that hard or surprising. Now, this isn't exactly a good thing as it could lead to high grade boundaries. Ah, it's 50/50. All of us would get As only in my dreams lol Anything could happen this year.
Original post by blueberry389
when you say sth is being oxidised or reduced, do you say the element of the compound or the compound?
e.g. the S in SO2 is reduced / SO2 is reduced?
thank you!
e.g. the S in SO2 is reduced / SO2 is reduced?
thank you!

The sulfur element is being reduced because you're talking about the oxidation state of sulfur only.
SO2 - O = -2 x 2 = -4 so S = +4

When I am talking about acidified potassium dichromate (IV) orange to green chromium (III) ions would I be right in saying the potassium dichromate is REDUCED to chromium ions?
Hi guys, I have produced a glossary of terms for F321 mod-1 (atoms and reactions) if you want to have a look. Thanks.
http://www.thestudentroom.co.uk/g/resources/ocr_chem_a_f321_module_1_atoms_and_reactions_glossary
http://www.thestudentroom.co.uk/g/resources/ocr_chem_a_f321_module_1_atoms_and_reactions_glossary
Hi 
I'm a tiny bit confused about the investigation for the ease of decomposition for Carbonates.
I understand the procedure.
But why are we using this apparatus? What are we observing? How long it takes for the lime water to become cloudy, due to the CO2 formed in the equation:
MCO3 --> MgO + CO2
Is the experiment for all carbonates?
I thought aqueous Ca(CO3) is lime water.. So why are we using this?
Lol ok maybe not a 'tiny' bit confused

I'm a tiny bit confused about the investigation for the ease of decomposition for Carbonates.
I understand the procedure.
But why are we using this apparatus? What are we observing? How long it takes for the lime water to become cloudy, due to the CO2 formed in the equation:
MCO3 --> MgO + CO2
Is the experiment for all carbonates?
I thought aqueous Ca(CO3) is lime water.. So why are we using this?
Lol ok maybe not a 'tiny' bit confused

Original post by Dinaa
Hi 
I'm a tiny bit confused about the investigation for the ease of decomposition for Carbonates.
I understand the procedure.
But why are we using this apparatus? What are we observing? How long it takes for the lime water to become cloudy, due to the CO2 formed in the equation:
MCO3 --> MgO + CO2
Is the experiment for all carbonates?
I thought aqueous Ca(CO3) is lime water.. So why are we using this?
Lol ok maybe not a 'tiny' bit confused

I'm a tiny bit confused about the investigation for the ease of decomposition for Carbonates.
I understand the procedure.
But why are we using this apparatus? What are we observing? How long it takes for the lime water to become cloudy, due to the CO2 formed in the equation:
MCO3 --> MgO + CO2
Is the experiment for all carbonates?
I thought aqueous Ca(CO3) is lime water.. So why are we using this?
Lol ok maybe not a 'tiny' bit confused

Yes it is how long it takes for the lime water to become cloudy to compare the time taken for decomposition between two carbonates.
Ca(OH)2 (aq) is lime water. When CO2 is passed through it reacts like this Ca(OH)2 (aq) + CO2 --> CaCO3 (s) + H2O. CaCO3 is the white precipitate.
Posted from TSR Mobile
Original post by samwillettsxxx
When I am talking about acidified potassium dichromate (IV) orange to green chromium (III) ions would I be right in saying the potassium dichromate is REDUCED to chromium ions?
Yah. The compound has been reduced, therefore reduction has occurred. We don't worry too much about the potassium, we treat it as a spectator ion.
I can see why you'd be hesitant to call it reduction, because that would imply that everything in the chromate (inc. the oxygen) is being reduced. It would be more accurate to say that the chromium in the chromate is being reduced, but saying that the 'chromate has been reduced' is still deemed more than acceptable.
Source: Bossed A2 Chem.
Original post by C0balt
Yes it is how long it takes for the lime water to become cloudy to compare the time taken for decomposition between two carbonates.
Ca(OH)2 (aq) is lime water. When CO2 is passed through it reacts like this Ca(OH)2 (aq) + CO2 --> CaCO3 (s) + H2O. CaCO3 is the white precipitate.
Posted from TSR Mobile
Ca(OH)2 (aq) is lime water. When CO2 is passed through it reacts like this Ca(OH)2 (aq) + CO2 --> CaCO3 (s) + H2O. CaCO3 is the white precipitate.
Posted from TSR Mobile
That's a superb explanation!
Thank you again

Original post by Dinaa
That's a superb explanation!
Thank you again
Thank you again

Np! Happy to help

Posted from TSR Mobile
I have been researching to try and find the correct way to convert a number from mol/dm^3 to g/dm^3 but I am really struggling. Which is the correct way to do it accurately?
Could you provide an exam so I can see how you do it.
thanks
Could you provide an exam so I can see how you do it.
thanks
Yes, me !
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- Chemistry Degree Lab Work
- Making chemistry notes
- Physical natural sciences
- !!! A level choices - Chem or Computer science
- Do I have enough time to turn my grades around??
- A level chemistry for a biology degree? (help please!!)
- Mass vs relative atomic mass on periodic table
- What is it like doing chem vs natural sciences at uni?
- Official NATURAL SCIENCES applicants thread 2024
- in need of urgent advice, would appreciate any help :)
- Lancaster uni timetable
- Study leave
- Need help on a Venn diagram question
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me?
- Higher chemistry
- Cambridge Natural Sciences Interview Applicants 2023/24
- Biochemistry at University
- How do I get grades 7-9?
Latest
Last reply 3 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 6 minutes ago
AQA GCSE English Literature Paper 1 (8702/1) - 13th May 2024 [Exam Chat]Last reply 8 minutes ago
Kingston or Westminster university for architecture?Last reply 12 minutes ago
Official: King's College London A100 2024 Entry ApplicantsMedical Schools
1020
Last reply 16 minutes ago
JK Rowling in ‘arrest me’ challenge over hate crime lawLast reply 20 minutes ago
NICS Staff Officer and Deputy Principal recruitment 2022 2023Last reply 26 minutes ago
Official: University of Manchester A106 2024 Entry ApplicantsMedical Schools
1292
Last reply 26 minutes ago
The Official King's College London Applicants for 2024 Entry ThreadLast reply 29 minutes ago
Woodhouse College applicants 2024Last reply 30 minutes ago
BAE systems degree apprenticeships September 2024



