why are these not counted as methyl groups?
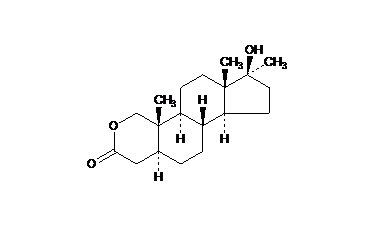
the CH3 groups on the diagram are not counted as methyl groups, how come?
Scroll to see replies
I count them as methyl groups, and that's all that should matter.
Original post by Straighthate
the CH3 groups on the diagram are not counted as methyl groups, how come?

the CH3 groups on the diagram are not counted as methyl groups, how come?
They are methyl groups. /thread
Original post by langlitz
They are methyl groups. /thread
in the mark scheme for a paper they are not
Original post by Straighthate
in the mark scheme for a paper they are not
Well, they are. So, either the mark scheme was wrong, or the question was worded such to preclude writing "Me" in place of "CH3", or they just decided to put the equivalent "CH3" as the answer.
If it doesn't say "Do NOT accept "Me" or an unlabelled terminal skeletal arm in place of a terminal "CH3" label" then I wouldn't be worried; it all means the same thing.
Original post by Straighthate
in the mark scheme for a paper they are not
And is this reasoned by your paper? as the people said it above, they are methyl groups. I can't see another ones.
Look at the mark scheme again
Original post by Infraspecies
Well, they are. So, either the mark scheme was wrong, or the question was worded such to preclude writing "Me" in place of "CH3", or they just decided to put the equivalent "CH3" as the answer.
If it doesn't say "Do NOT accept "Me" or an unlabelled terminal skeletal arm in place of a terminal "CH3" label" then I wouldn't be worried; it all means the same thing.
If it doesn't say "Do NOT accept "Me" or an unlabelled terminal skeletal arm in place of a terminal "CH3" label" then I wouldn't be worried; it all means the same thing.
But why functional groups like methyl groups should be precluded or regarded as equivalent, if they exist?
Original post by Kallisto
But why functional groups like methyl groups should be precluded or regarded as equivalent, if they exist?
I don't know what you're trying to say, reword that please.
They are methyl groups - if the markscheme says otherwise... then its wrong
Original post by Infraspecies
I don't know what you're trying to say, reword that please.
Then I have not understand your statement before. I was confused to be honest. What did you mean when you say 'to put the equivalent "CH3" as the answer'? that CH3 should not regarded as a functional group by a paper? I don't get it.
Original post by Kallisto
And is this reasoned by your paper? as the people said it above, they are methyl groups. I can't see another ones.
http://www.ocr.org.uk/Images/135165-mark-scheme-unit-f324-rings-polymers-and-analysis-june.pdf
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
Original post by Straighthate
http://www.ocr.org.uk/Images/135165-mark-scheme-unit-f324-rings-polymers-and-analysis-june.pdf
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
Methyl groups arent fuctional groups
Functional groups are responsible for the reactions of a compound. Eg alkene
Posted from TSR Mobile
Original post by brother aldi
Methyl groups arent fuctional groups
Functional groups are responsible for the reactions of a compound. Eg alkene
Posted from TSR Mobile
Functional groups are responsible for the reactions of a compound. Eg alkene
Posted from TSR Mobile
****
Original post by Straighthate
http://www.ocr.org.uk/Images/135165-mark-scheme-unit-f324-rings-polymers-and-analysis-june.pdf
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
See, you took the question out of context. When a question asks you to name the functional groups, you're not supposed to name every single group of atoms in the molecule, only the "special" or specific ones that give the molecule its particular properties and/or reactivity.
So for this molecule I'd say the functional groups are the lactone and the alcohol.
(edited 8 years ago)
Original post by Straighthate
http://www.ocr.org.uk/Images/135165-mark-scheme-unit-f324-rings-polymers-and-analysis-june.pdf
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
Yeah, but I don't get it why this should be wrong. The whole molecule has three CH3-group and one OH-group. And I can't see a plausible reasoning.
But perhaps these groups are regarded just as parts of the molecule? no, that is senseless.
EDIT: okay, I have read the posted statements above. So I see the sense.
(edited 8 years ago)
Original post by Straighthate
http://www.ocr.org.uk/Images/135165-mark-scheme-unit-f324-rings-polymers-and-analysis-june.pdf
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
question 3ci, it asks you what functional groups are present in this molecule, as shown by the mark scheme you LOSE mark if you mention that there is a methyl group
I also think it's slightly oversimplified to call the C=O next to the O just an ester, when it's actually a lactone (cyclic ester). A lactone is more reactive than a normal ester because in a normal ester there is additional stabilisation in the Z conformation provided by the overlap of the oxygen lone pair and the C-O sigma anti bonding orbital of the C=O bond.
(edited 8 years ago)
Original post by brother aldi
Methyl groups arent fuctional groups
Functional groups are responsible for the reactions of a compound. Eg alkene
Functional groups are responsible for the reactions of a compound. Eg alkene
Just to hedge my bet: functional groups in the narrower sense are the ones who are reacting with each other and forming a bound between molecules? so the NH2- and COOH-group of amino acids in charged states for instance?
Original post by EdmundWoodstock
I also think it's slightly oversimplified to call the C=O next to the O just an ester, when it's actually a lactone (cyclic ester). A lactone is more reactive than a normal ester because in a normal ester there is additional stabilisation in the Z conformation provided by the overlap of the oxygen lone pair and the C-O sigma anti bonding orbital of the C=O bond.
thi is A2 though
Original post by Straighthate
thi is A2 though
I know, sorry, I just can't resist correcting errors or vagueness, given I'm studying Natural Sciences at Uni

(edited 8 years ago)
Quick Reply
Related discussions
- chem isomer as help?
- How come this isn't written as 2,2-dimethyl-4-chlorobutanal
- A level Chemistry OCR HELP
- Nucleophilic substitution - Need help
- Help chemistry
- A level chemistry optical isomerism MC questions
- is but-1-ene the same as butene
- A level Chemistry help
- Amount of substance chemistry
- Boiling point of halogenoalkanes
- Spectroscopy help
- Organic chemistry help
- Skeletal Formulae
- Which of the functional groups consist only of carbon and hydrogen?
- Why is1-methylpentane not an isomer of Hexane
- Can you substitute in positions 2 and 4 even if the directing group is 3 directing
- Indicator colour question.
- AQA Chemistry Alevel Organic synthesis
- Urgent help chemistry
- Esterification
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



