AS Chemistry- helping each other out!
Scroll to see replies
Original post by TeaAndTextbooks
Can someone explain the electrophillic addition of bromine water to cyclopentene? Thanks
Posted from TSR Mobile
Posted from TSR Mobile
I think this is how you do it. I may have an arrow wrong way round though, I don't think I do though, maybe someone can verify?
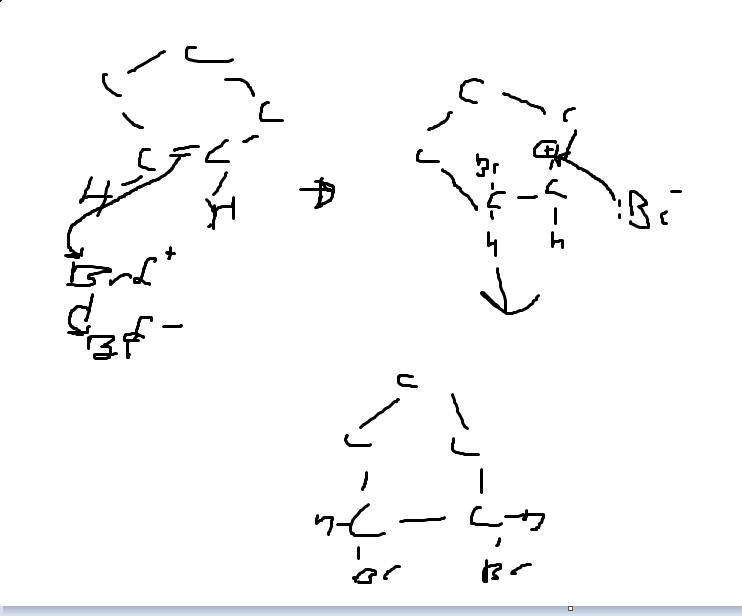
Original post by TheNoobishKnight
I think this is how you do it. I may have an arrow wrong way round though, I don't think I do though, maybe someone can verify?


Love it
Original post by C0balt
Love it
lol thanks doing bio revision atm so had to do it quick :P.
So much darn mechanisms i keep confusing them with A2 stuff lol.
Thank you so much, I really needed a diagram and I appreciate this art work lol 😁
Posted from TSR Mobile
Posted from TSR Mobile
Original post by NotoriousS
Yes I am
It's tomorrow! :-(
im reallly scared im doing edexcel unit 1 and apparently its gonna be the R paper
Did anyone else think the AQA EMPA was really really hard?
Original post by Rstlss
me
hiiii
Original post by LibertyMan
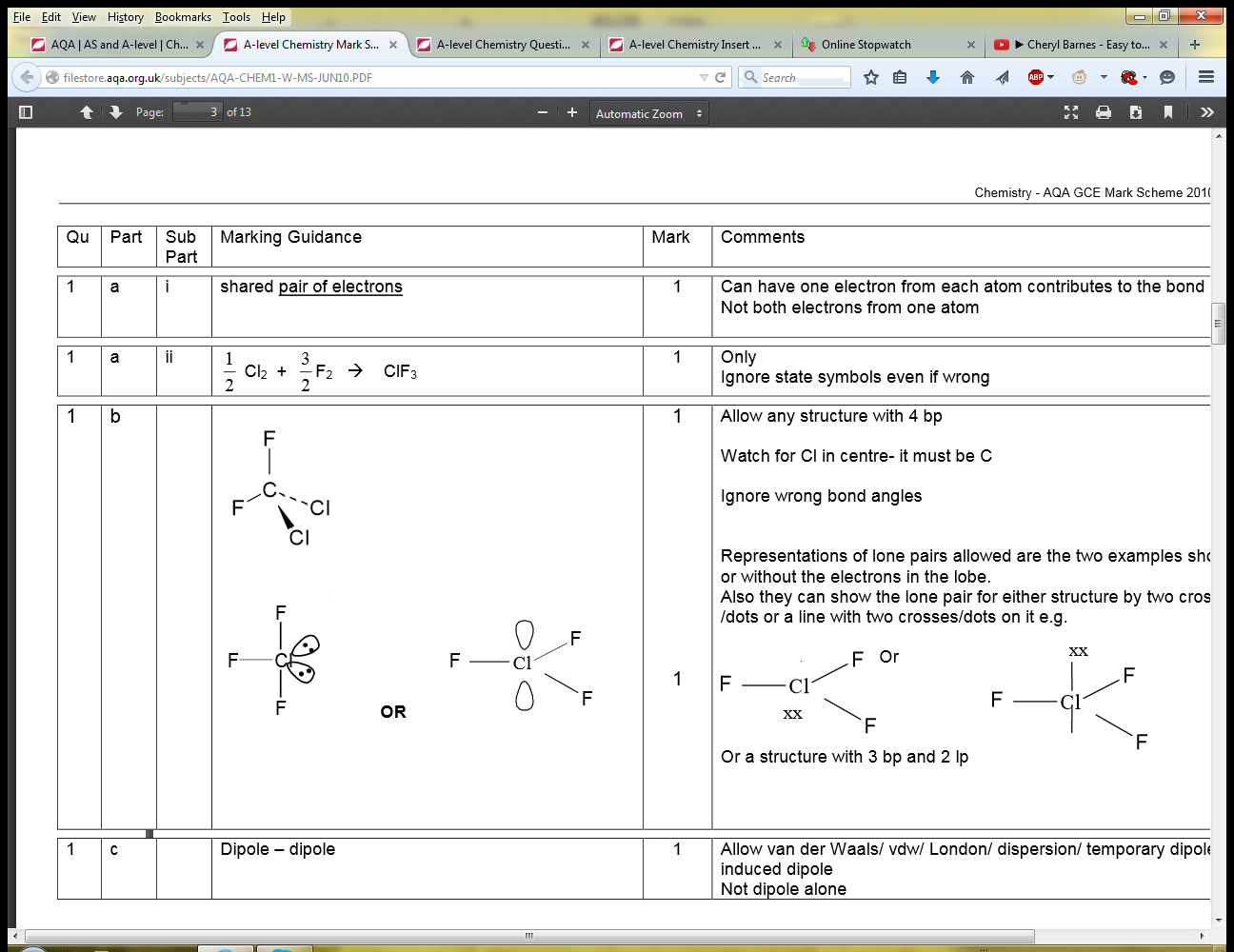
Can someone explain why dipole dipole is the answer
--> If this was NH4+, or CH4, there wouldn't be any dipoles because the molecule is surrounded by the same delta charge, so another molecule cannot be attracted to it as the like charges on the outside of each molecule will repel them away.
However, with CCl2F2, there is dipole-dipole formation. Why? Is it because of the polarity difference between C-F and C-Cl?

Can someone explain why dipole dipole is the answer
--> If this was NH4+, or CH4, there wouldn't be any dipoles because the molecule is surrounded by the same delta charge, so another molecule cannot be attracted to it as the like charges on the outside of each molecule will repel them away.
However, with CCl2F2, there is dipole-dipole formation. Why? Is it because of the polarity difference between C-F and C-Cl?
And it burns, burns, burns (tabs!)
Original post by Mr.bob
And it burns, burns, burns (tabs!)
I'll burn you if you don't answer my question
Original post by Shadowfire123
hiiii
yeah?
Original post by Shadowfire123
im reallly scared im doing edexcel unit 1 and apparently its gonna be the R paper
What's the R paper? any difference?
Original post by NotoriousS
What's the R paper? any difference?
International paper, same content but slightly harder for some reason
Any likely Q's ???
Im really dreading this paper
Im really dreading this paper
Could anyone tell me what chemical tests we may need to know? AS Edexcel unit 1
Original post by NotoriousS
What's the R paper? any difference?
How do you know this?
Can someone explain dative covalent bonding I have never really got it.
like how do you work out if it is a dative covalent bond and which element is giving the lone pair of electrons
can someone explain using phosphine PH3 to form PH4+.
The specific question is to give the name of the type of bond formed when phosphine reacts with an H+ ion. Explain how it is formed
like how do you work out if it is a dative covalent bond and which element is giving the lone pair of electrons
can someone explain using phosphine PH3 to form PH4+.
The specific question is to give the name of the type of bond formed when phosphine reacts with an H+ ion. Explain how it is formed
Original post by allysid123
does anyone know why the answer to this question is A and not C?
In which of the following series of elements is there an increase in the melting temperatures from left to right?
A) Na Mg Al
B) Li Na K
C)B C N
D) Si P S
In which of the following series of elements is there an increase in the melting temperatures from left to right?
A) Na Mg Al
B) Li Na K
C)B C N
D) Si P S
carbon has a much higher melting temperature than nitrogen...
Folks what is the ideal definition of Avogadro constant?
Original post by DecentGuy
Folks what is the ideal definition of Avogadro constant?
In the OCR A glossary is "The number of atoms per mole"
Quick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- Chemistry Degree Lab Work
- Making chemistry notes
- Physical natural sciences
- !!! A level choices - Chem or Computer science
- Do I have enough time to turn my grades around??
- A level chemistry for a biology degree? (help please!!)
- Mass vs relative atomic mass on periodic table
- What is it like doing chem vs natural sciences at uni?
- Official NATURAL SCIENCES applicants thread 2024
- in need of urgent advice, would appreciate any help :)
- Lancaster uni timetable
- Study leave
- Need help on a Venn diagram question
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me?
- Higher chemistry
- Cambridge Natural Sciences Interview Applicants 2023/24
- Biochemistry at University
- How do I get grades 7-9?
Latest
Last reply 2 minutes ago
Official: King's College London A100 2024 Entry ApplicantsMedical Schools
1021
Last reply 2 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 4 minutes ago
Official University of St Andrews Applicant Thread for 2024Last reply 8 minutes ago
Official University College London Applicant Thread for 2024Posted 9 minutes ago
Occupational therapy wolverhampton universityLast reply 13 minutes ago
Does LSE accept students who reapply?Last reply 37 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2868
Last reply 37 minutes ago
Is meeting potential partners through friends the most vaible strategy?Last reply 37 minutes ago
Is anyone going to graphic design in 2024 in university of Northampton?Last reply 42 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsPosted 47 minutes ago
Not reporting to Universal credit my maintenance loan amount!!



