AS Chemistry Help
I am having trouble understanding one of the January 2011 questions.It's Question 5b. The mark scheme is confusing, any help would be appreciated 

Bump
Post question or Link on Here and i'll give it a stab
Posted from TSR Mobile
Posted from TSR Mobile
Original post by GrappleX
http://www.ocr.org.uk/Images/65360-question-paper-unit-f322-chains-energy-and-resources.pdf
It's Question 5b :/
Original post by HoangLe
What is your exam board?
OCR
...anyone? D:

Firstly they give us a table so lets look at that:
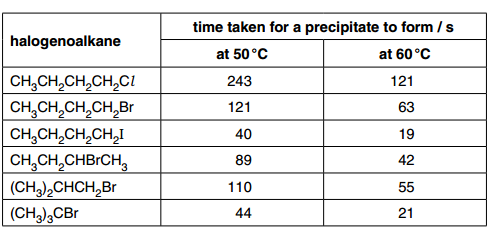
The first bullet point specifies we need to talk about the halogens in the halogenoalkanes (The first column) so lets just glance through that.
You should notice in the first 3 the halogens in the halogenoalkanes are all different and this affects the results as you can see at 50 degrees that it takes longer the Cl Halogenoalkane to precipitate longer than the Br and the Br takes longer to precipitate than the I halogenoalkane.
So here you can say the chloroalkane reacts the slowest and the iodoalkane reacts the faster (as shown in the table).
Then you need to justify why and this is where your chemistry knowledge comes in:
You need to know the context of the bond strengths of halogenoalkanes.
Simply: The C - Cl bond is stronger than the C - Br bond and this bond is stronger than the C - I bond.
In summary C - Cl > C - Br > C - I
If you want to know why look here:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
So you can now explain that the Chloroalkane takes longer to precipitate because of their bond strengths and here you go into detail for an example and say something along the lines of :
The C - I bond is weaker than the C - Br bond
The C - Br bond is weaker than the C - Cl Bond
-----------------------------------------------------------------------------------------------------------------
Second bullet point
This is knowledge based as well:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
http://www.chemguide.co.uk/mechanisms/nucsub/water.html#top
Basically you need to know that a tertiary alcohol hydrolyses faster than a secondary and the secondary hydrolyses faster than primary.
The links above explain it but in simple terms its all in the mechanism. The links I posted should hopefully explain it.
-----------------------------------------------------------------------------------------------------------------
Third bullet point
Here you state that the halogenalkanes rate of reaction increases as the temperature increases and give evidence e.g :
The chlroalkane took 243 seconds to precipitate at 50 degrees but 121 seconds at 60 degrees
Then you use your chemistry knowledge that high temperatures means more kinetic energy and so more successful collisions (Just like the Boltzmann Distribution diagram)
(edited 8 years ago)
Original post by HoangLe
Firstly they give us a table so lets look at that:

The first bullet point species we need to talk about the halogens in the halogenoalkanes (The first column) so lets just glance through that.
You should notice in the first 3 the halogens in the halogenoalkanes are all different and this affects the results as you can see at 50 degrees that it takes longer the Cl Halogenoalkane to precipitate longer than the Br and the Br takes longer to precipitate than the I halogenoalkane.
So here you can say the chloroalkane reacts the slowest and the iodoalkane reacts the faster (as shown in the table).
Then you need to justify why and this is where your chemistry knowledge comes in:
You need to know the context of the bond strengths of halogenoalkanes.
Simply: The C - Cl bond is stronger than the C - Br bond and this bond is stronger than the C - I bond.
In summary C - Cl > C - Br > C - I
If you want to know why look here:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
So you can now explain that the Chloroalkane takes longer to precipitate because of their bond strengths and here you go into detail for an example and say something along the lines of :
The C - I bond is weaker than the C - Br bond
The C - Br bond is weaker than the C - Cl Bond
-----------------------------------------------------------------------------------------------------------------
Second bullet point
This is knowledge based as well:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
http://www.chemguide.co.uk/mechanisms/nucsub/water.html#top
Basically you need to know that a tertiary alcohol hydrolyses faster than a secondary and the secondary hydrolyses faster than primary.
The links above explain it but in simple terms its all in the mechanism. The links I posted should hopefully explain it.
-----------------------------------------------------------------------------------------------------------------
Third bullet point
Here you state that the halogenalkanes rate of reaction increases as the temperature increases and give evidence e.g :
The chlroalkane took 243 seconds to precipitate at 50 degrees but 121 seconds at 60 degrees
Then you use your chemistry knowledge that high temperatures means more kinetic energy and so more successful collisions (Just like the Boltzmann Distribution diagram)

Firstly they give us a table so lets look at that:

The first bullet point species we need to talk about the halogens in the halogenoalkanes (The first column) so lets just glance through that.
You should notice in the first 3 the halogens in the halogenoalkanes are all different and this affects the results as you can see at 50 degrees that it takes longer the Cl Halogenoalkane to precipitate longer than the Br and the Br takes longer to precipitate than the I halogenoalkane.
So here you can say the chloroalkane reacts the slowest and the iodoalkane reacts the faster (as shown in the table).
Then you need to justify why and this is where your chemistry knowledge comes in:
You need to know the context of the bond strengths of halogenoalkanes.
Simply: The C - Cl bond is stronger than the C - Br bond and this bond is stronger than the C - I bond.
In summary C - Cl > C - Br > C - I
If you want to know why look here:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
So you can now explain that the Chloroalkane takes longer to precipitate because of their bond strengths and here you go into detail for an example and say something along the lines of :
The C - I bond is weaker than the C - Br bond
The C - Br bond is weaker than the C - Cl Bond
-----------------------------------------------------------------------------------------------------------------
Second bullet point
This is knowledge based as well:
http://www.chemguide.co.uk/organicprops/haloalkanes/agno3.html#top
http://www.chemguide.co.uk/mechanisms/nucsub/water.html#top
Basically you need to know that a tertiary alcohol hydrolyses faster than a secondary and the secondary hydrolyses faster than primary.
The links above explain it but in simple terms its all in the mechanism. The links I posted should hopefully explain it.
-----------------------------------------------------------------------------------------------------------------
Third bullet point
Here you state that the halogenalkanes rate of reaction increases as the temperature increases and give evidence e.g :
The chlroalkane took 243 seconds to precipitate at 50 degrees but 121 seconds at 60 degrees
Then you use your chemistry knowledge that high temperatures means more kinetic energy and so more successful collisions (Just like the Boltzmann Distribution diagram)
THANK YOU SO MUCH! I actually understand it a lot more clearly now
 I wish I could rep, I ran out
I wish I could rep, I ran out 
Quick Reply
Related discussions
- GCSE Exam Discussions 2024
- Chemistry at Uni.
- Undergraduate chemistry
- A level chemistry for a biology degree? (help please!!)
- pls be my chemistry tutor !!
- What is actually studied in a chemistry degree?
- chemistry or psychology a level??
- TSR Study Together - STEM vs Humanities!
- Ask a Chemistry Uni Student!
- Biochemistry at University
- Best extracurriculars for Oxford uni?
- Biomed vs Biochem vs chemistry
- A Level Choices Help (Aqa Physics V Chemistry)
- A level help
- Workload of Chemistry/Natural sciences degree
- advanced higher chemistry help
- I don't understand chemistry :'(
- Really confused about my a level options
- Making uni choices
- How to make my application strong
Latest
Last reply 1 minute ago
LSE anthropology and law 2024Last reply 2 minutes ago
Official: University of Bristol A100 2024 Entry ApplicantsLast reply 3 minutes ago
economic history lse offersLast reply 3 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Last reply 3 minutes ago
Official University of Edinburgh Applicant Thread for 2024Last reply 11 minutes ago
UAL Bsc Fashion Management and fashion marketingLast reply 12 minutes ago
Anybody interested in sitting the Hong Kong Medical Licensing Exam?Last reply 14 minutes ago
AQA A Level Spanish Paper 1 7692/1 - 7th June 2023 [Exam Chat]Last reply 20 minutes ago
Official: University of Birmingham A100 2024 Entry ApplicantsMedical Schools
1039
Last reply 25 minutes ago
Official Politics and/or International Relations Applicants Thread 2024Last reply 27 minutes ago
Economics at Edinburgh vs Manchester vs LSE (Ispp & E) for IBTrending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]Trending
Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 1 week ago
AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]Posted 2 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Last reply 3 months ago
OCR A GCSE Chemistry Paper 2 (Higher Combined) J250/10- 13th June [Exam Chat]



