AQA Physics PHYA5 - Thursday 18th June 2015 [Exam Discussion Thread]
Scroll to see replies
Anyone got a link for the old spec past papers?
Posted from TSR Mobile
Posted from TSR Mobile
Original post by BoigaDendrophila
They're in the first post

Posted from TSR Mobile
What does it mean by certain radioactive isotopes are easier to screen? Where can I find information on this in the nelson thornes book
Original post by AR_95
What does it mean by certain radioactive isotopes are easier to screen? Where can I find information on this in the nelson thornes book
I assume it's referring to the various penetrating powers (of alpha, beta +-, gamma) of the radioactive emissions. For example, alpha would be easier to screen than gamma because alpha has a lower penetrating power.
Original post by AR_95
What does it mean by certain radioactive isotopes are easier to screen? Where can I find information on this in the nelson thornes book
Original post by PotterPhysics
I assume it's referring to the various penetrating powers (of alpha, beta +-, gamma) of the radioactive emissions. For example, alpha would be easier to screen than gamma because alpha has a lower penetrating power.
Yeah screening is an alternative to "shielding"

Hence why lead aprons are often worn etc.
Posted from TSR Mobile
Original post by CD223
Yeah screening is an alternative to "shielding" 
Hence why lead aprons are often worn etc.
Posted from TSR Mobile

Hence why lead aprons are often worn etc.
Posted from TSR Mobile
That makes sense.
In the cgp book it says that a nuclear reactor is surrounded by a thick concrete casing which acts as a shield. Nuclear fission produces gamma radiation. On another page, it says that gamma radiation can be blocked by several meters of concrete. Does that mean nuclear reactors are cased in several meters of concrete?! (Edit: What I was thinking is it would be more practical (due to size considerations, etc.) to use a lead casing, as then it would only be a few cm thick.)
(edited 8 years ago)
Original post by CD223
Does anyone know when you do and don't you take into account the mass of a particle/atom when considering binding energy? Is it when they're on their own? Ie: 1 electron/neutron/proton?
Posted from TSR Mobile
Posted from TSR Mobile
You can't have a binding energy between 1 thing. I considered a hadron comprised of quarks but they can't be classed as binding energy due to gluons and whatnot, anyway, tangent.
The binding energy is the energy required to break up a nucleus into its consistent protons and neutrons. Hence, the mass difference times c² is the binding energy. We always account for the mass for a particle. It's important to note for these questions you use the different masses for the proton and neutron unless otherwise stated (as the 3rd decimal place is important). If a particle is solitary binding energy=0 as it cannot be broken down further

Posted from TSR Mobile
Funny enough I was talking about the topic yesterday but...
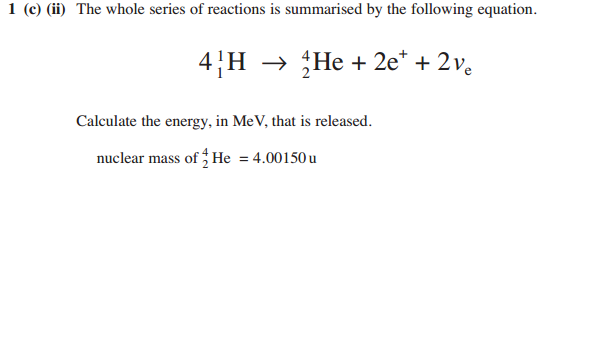
How am I supposed to work out the mass of the left hand side ffs
EDIT :
Nevermind I didn't realise it was in the data sheet
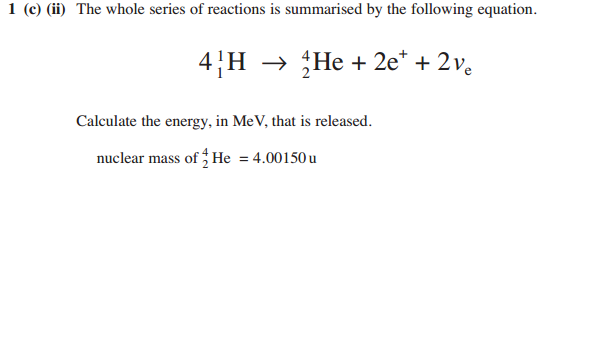
How am I supposed to work out the mass of the left hand side ffs
EDIT :
Nevermind I didn't realise it was in the data sheet
(edited 8 years ago)
Original post by AR_95
Funny enough I was talking about the topic yesterday but...
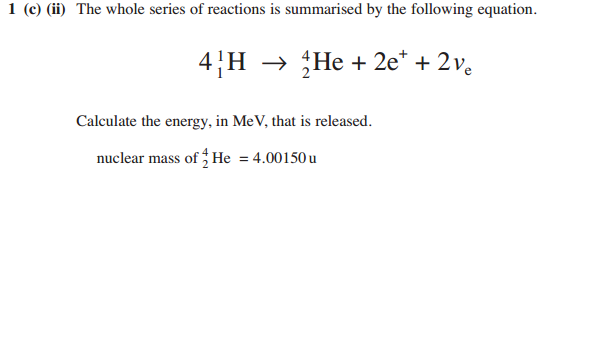
How am I supposed to work out the mass of the left hand side ffs
EDIT :
Nevermind I didn't realise it was in the data sheet
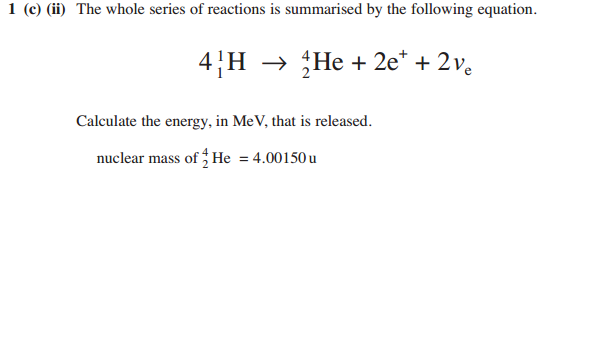
How am I supposed to work out the mass of the left hand side ffs
EDIT :
Nevermind I didn't realise it was in the data sheet
Energy is released from the nucleus so we only need to consider nuclear mass. Nuclear mass of left hand side is just 4 times the mass of a hydrogen nucleus, that is, 4 times the mass of a proton, i.e. 4u. This is because a lone proton doesn't have any mass defect.
Regarding edit: Is my answer incorrect?
Oh wait, yes, sorry. The proton rest mass isn't exactly 1u, we have to take it up to 5 decimal places since that is what the He one is given to. Silly oversight

(edited 8 years ago)
Original post by PotterPhysics
Energy is released from the nucleus so we only need to consider nuclear mass. Nuclear mass of left hand side is just 4 times the mass of a hydrogen nucleus, that is, 4 times the mass of a proton, i.e. 4u. This is because a lone proton doesn't have any mass defect.
Regarding edit: Is my answer incorrect?
Regarding edit: Is my answer incorrect?
4u yes but you're not supposed to consider it as 4u. I realised it was 4 protons beforehand, but I couldn't find anywhere that the mass of proton = 1.00728U which is usually given in these types of questions. After a while and constant staring at the formula sheet, I found that the mass (1.00728u) for proton is actually in the list of all the constants (it's just under proton rest mass). The same is given for neutron and electron which is quite frankly wonderful
Original post by AR_95
Funny enough I was talking about the topic yesterday but...
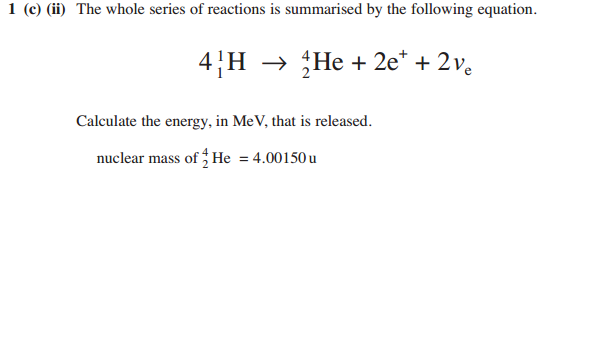
How am I supposed to work out the mass of the left hand side ffs
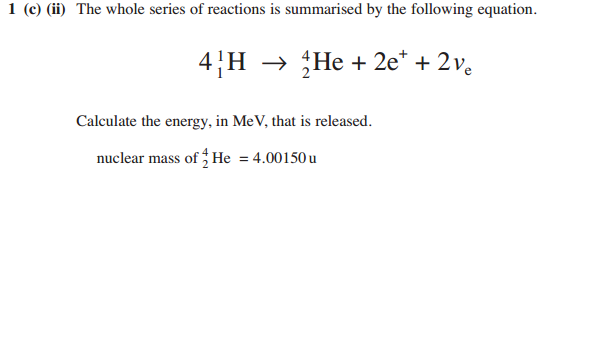
How am I supposed to work out the mass of the left hand side ffs
Assume that the electron neutrino has zero mass.
u is a constant. It would be wise to convert to kg for simplicity.
u=1.661x10^-27
Mass after=(4.00150u=6.646915x10-27kg)+2(9.11x10-31)
Mass before=4(1.673x10-27)
change in mass=4.3263x10-29
E=mc^2
E=3.89367x10-12J
Converting to electron Volts is to divide by the magnitude charge of the electron (1.60x10-19)
In MeV=24.34MeV
(edited 8 years ago)
It took so long for me to type I didn't see you'd solved 

Btw I saw and have done most of the past papers from the "OLD PPQ's" link, however I did see some people mentioning 2002/2003 questions. where are these from?
Original post by Amanzz
Assume that the electron neutrino has zero mass.
u is a constant. It would be wise to convert to kg for simplicity.
u=1.661x10^-27
Mass after=(4.00150u=6.646915x10-27kg)+2(9.11x10-31)
Mass before=4(1.673x10-27)
change in mass=4.3263x10-29
E=mc^2
E=3.89367x10-12J
Converting to electron Volts is to divide by the magnitude charge of the electron (1.60x10-19)
In MeV=24.34MeV
u is a constant. It would be wise to convert to kg for simplicity.
u=1.661x10^-27
Mass after=(4.00150u=6.646915x10-27kg)+2(9.11x10-31)
Mass before=4(1.673x10-27)
change in mass=4.3263x10-29
E=mc^2
E=3.89367x10-12J
Converting to electron Volts is to divide by the magnitude charge of the electron (1.60x10-19)
In MeV=24.34MeV
I always knew how to do the question. My only problem (a small one) was finding the value of Mp (mass of proton in U) which I found was given on the formula sheet
Your way of doing it brings you close to the final answer but it wouldn't of been accepted in the mark scheme, which specifies 24.7 MeV
The way I did it/mark scheme suggested was
(4x1.00728u) - [ (4.00150u) + ( 2 x 5.4x10^-4u)
Which gave a remainder of 0.02652 u
Simply multiplying it by 931.3 gives 24.69 IE 24.7
I think you'd get 1 or 2 marks for the right method though
(edited 8 years ago)
Do we use 931.3MeVu^-1 (as given in teh cgp book) or 931.5MeVu^-1 (as given in the aqa data booklet)? I'm thinking the latter but everyone uses 931.3...
Also guys I just want reassurance. Heard a dreadful rumour from my teacher last year that they weren't giving you an equations sheet in the exam this year? Is that even remotely true?
Original post by PotterPhysics
Do we use 931.3MeVu^-1 (as given in teh cgp book) or 931.5MeVu^-1 (as given in the aqa data booklet)? I'm thinking the latter but everyone uses 931.3...
Oh wow, I didn't even realise this haha. The mark scheme for that question included 931.5 initially, but in brackets next to it said allow 931.3. In this question using both values you still end up rounding to 24.7 so it wouldn't have mattered. I think both are just as acceptable but I'll keep an eye out from now on, on what they prefer.
(edited 8 years ago)
Original post by AR_95
Also guys I just want reassurance. Heard a dreadful rumour from my teacher last year that they weren't giving you an equations sheet in the exam this year? Is that even remotely true?
I highly doubt it they would suddenly change the data booklet when the specification is the same. Perhaps he was referring to next year's new spec?
Original post by AR_95
Also guys I just want reassurance. Heard a dreadful rumour from my teacher last year that they weren't giving you an equations sheet in the exam this year? Is that even remotely true?
Nope, completely wrong. They may be removing them or decreasing how much is on them for the new syllabus (I've heard rumours), but nothing to affect us.
Original post by PotterPhysics
That makes sense.
In the cgp book it says that a nuclear reactor is surrounded by a thick concrete casing which acts as a shield. Nuclear fission produces gamma radiation. On another page, it says that gamma radiation can be blocked by several meters of concrete. Does that mean nuclear reactors are cased in several meters of concrete?! (Edit: What I was thinking is it would be more practical (due to size considerations, etc.) to use a lead casing, as then it would only be a few cm thick.)
In the cgp book it says that a nuclear reactor is surrounded by a thick concrete casing which acts as a shield. Nuclear fission produces gamma radiation. On another page, it says that gamma radiation can be blocked by several meters of concrete. Does that mean nuclear reactors are cased in several meters of concrete?! (Edit: What I was thinking is it would be more practical (due to size considerations, etc.) to use a lead casing, as then it would only be a few cm thick.)
Yeah they use concrete.
Purely due to structural engineering it's less practical to use lead due to container fatigue etc.
Posted from TSR Mobile
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- GCSE Exam Discussions 2023
- OCR B A-level Physics Paper 2 Advancing Physics (H557/02) - 9th June 2023 [Exam Chat]
- AQA GCSE Physics Paper 2 (Foundation Combined) 8464/2F - 16th June 2023 [Exam Chat]
- AQA GCSE Physical sciences (Higher Combined) 8465/4H - 13th June 2023 [Exam Chat]
- OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]
- OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]
- OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]
- GCSE exam advice
- AQA A Level Physical Education Paper 1 7582/1 - 26 May 2022 [Exam Chat]
- AQA GCSE Food Preparation and Nutrition Paper 1 (8585/W) - 19th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 2 (8035/2) - 5th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 3 (8035/3) - 14th June 2024 [Exam Chat]
- GCSE 2024 timetable
- AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]
- AQA GCSE Food Preparation and Nutrition - Tuesday 19th June 2024 [Exam Chat]
- English Lit Paper 2 leak??!?!!
Latest
Trending
Last reply 2 hours ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2024 [Exam Chat]Last reply 1 day ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]Trending
Last reply 2 hours ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2024 [Exam Chat]Last reply 1 day ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]



