OCR B Salter's Chemistry by Design F335 - 15th June 2015
Scroll to see replies
Original post by davvv
As I'm sure you all know F334 was a bloodbath. I worry that F335 is going to be just as awful. Does anyone have any advice on how to comprehend the questions quickly? You can sit there and read the question through but it saps precious time so there's a trade-off. Also is there anything in particular I should focus my revision on over the weekend apart from just stuff I'm not sure about?
F335 is like the entire chemistry course in one paper...
so much to learn esp the reactions..
Original post by suyoof123
F335 is like the entire chemistry course in one paper...
so much to learn esp the reactions..
so much to learn esp the reactions..
Indeed, but I've always preferred it to F334 for some reason. Think it's because there's more synoptic stuff from AS. I've made a few last-minute organic reaction cards, just gonna be testing myself with those at random points until the exam. Hopefully it'll stick.
Original post by davvv
As I'm sure you all know F334 was a bloodbath. I worry that F335 is going to be just as awful. Does anyone have any advice on how to comprehend the questions quickly? You can sit there and read the question through but it saps precious time so there's a trade-off. Also is there anything in particular I should focus my revision on over the weekend apart from just stuff I'm not sure about?
Generally I read the actual question before reading the context because then I can make the links whilst reading it, then I answer the questions
Posted from TSR Mobile
Original post by suyoof123
F335 is like the entire chemistry course in one paper...
so much to learn esp the reactions..
so much to learn esp the reactions..
Lol the reactions are killing me but i memorised it by singing it in the tune of the element song.
I'm sensing that a disgusting nmr spectrum is awaiting us
Original post by EggsterminateMe
Lol the reactions are killing me but i memorised it by singing it in the tune of the element song.
Dont be shy, whats the lyrics ^^
Its the reagants that are deadly imo.
Original post by suyoof123
Dont be shy, whats the lyrics ^^
Its the reagants that are deadly imo.
Its the reagants that are deadly imo.
Yes lol It's not all that and the reagents just come to me when I say the name. It's only the benzene ring reactions.
*Cough Cough*
# There is Acylation, Alkylation, Bromination, Chlorination, Hydrogenation, Nitration, Reduction, and Sul-fo-nation!#
Just ordered it in alphabetical order lol.
(The mini font is what i say in ym head when im Singing it. No kidding.
Probs missed a few things.
There is Acylation (RCOCL, AlCl3 catalyst, reflux) Alkylation (AlCl3, RCl, Reflux)
Bromination (Br2, FeBr2, room temp) Chlorination (Cl2, AlCl3 catalyst, warm)
Hydrogenation (H2, 300 degrees, 30 atm) Nitration(Conc. H2SO4, below 55 degrees) Reduction(Add Sn and HCl to Nitrobenzene) and Sul-fo-nation! (Conc H2SO4) #
Original post by EggsterminateMe
Yes lol It's not all that and the reagents just come to me when I say the name. It's only the benzene ring reactions.
*Cough Cough*
# There is Acylation, Alkylation, Bromination, Chlorination, Hydrogenation, Nitration, Reduction, and Sul-fo-nation!#
Just ordered it in alphabetical order lol.
(The mini font is what i say in ym head when im Singing it. No kidding.
Probs missed a few things.
There is Acylation (RCOCL, AlCl3 catalyst, reflux) Alkylation (AlCl3, RCl, Reflux)
Bromination (Br2, FeBr2, room temp) Chlorination (Cl2, AlCl3 catalyst, warm)
Hydrogenation (H2, 300 degrees, 30 atm) Nitration(Conc. H2SO4, below 55 degrees) Reduction(Add Sn and HCl to Nitrobenzene) and Sul-fo-nation! (Conc H2SO4) #
*Cough Cough*
# There is Acylation, Alkylation, Bromination, Chlorination, Hydrogenation, Nitration, Reduction, and Sul-fo-nation!#
Just ordered it in alphabetical order lol.
(The mini font is what i say in ym head when im Singing it. No kidding.
Probs missed a few things.
There is Acylation (RCOCL, AlCl3 catalyst, reflux) Alkylation (AlCl3, RCl, Reflux)
Bromination (Br2, FeBr2, room temp) Chlorination (Cl2, AlCl3 catalyst, warm)
Hydrogenation (H2, 300 degrees, 30 atm) Nitration(Conc. H2SO4, below 55 degrees) Reduction(Add Sn and HCl to Nitrobenzene) and Sul-fo-nation! (Conc H2SO4) #
WHOOP WHOOP

Is it Febr2 or FeBr3
https://en.wikipedia.org/wiki/Iron%28III%29_bromide
And then you got the alkane-alkene-carboxylic acids- amine .....
Original post by tealover96
I'm sensing that a disgusting nmr spectrum is awaiting us
I think so too, I'm absolutely dreading it. I think my tactic is going to be spend a couple minutes on it and if I'm not getting anywhere then to skip it and come back at the end.
Original post by suyoof123
WHOOP WHOOP 
Is it Febr2 or FeBr3
https://en.wikipedia.org/wiki/Iron%28III%29_bromide
And then you got the alkane-alkene-carboxylic acids- amine .....

Is it Febr2 or FeBr3
https://en.wikipedia.org/wiki/Iron%28III%29_bromide
And then you got the alkane-alkene-carboxylic acids- amine .....
Yes FeBr3 sorry.
I don't how I'm gonna learn those. Maybe use my mind palace somehow
Original post by EggsterminateMe
Yes FeBr3 sorry.
I don't how I'm gonna learn those. Maybe use my mind palace somehow
I don't how I'm gonna learn those. Maybe use my mind palace somehow
All that extra story line bs about van gough and his useless painting is annnoying.
WHO CARES ABOUT MORDANTING AND MONSTRAL BLUEEE"!!!£!"£
People are their 'interests'...
Original post by suyoof123
All that extra story line bs about van gough and his useless painting is annnoying.
WHO CARES ABOUT MORDANTING AND MONSTRAL BLUEEE"!!!£!"£
People are their 'interests'...
WHO CARES ABOUT MORDANTING AND MONSTRAL BLUEEE"!!!£!"£
People are their 'interests'...
I don't have the storylines book. It's a very boring one. Hopefully I can find all the important content on the internet
Original post by EggsterminateMe
I don't have the storylines book. It's a very boring one. Hopefully I can find all the important content on the internet
Dont many ppl take f335, this thread is dead.
We need to bloat it so more people are here to impart their knowledge

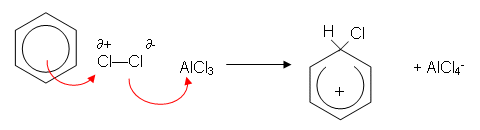
Like here, what happens first.
A dipole is induced on chlorine by benzene, so the negative chloride reacts with alcl4?
and the positive chloride then accepts a pair of electrons from benzene to form a covalent bond?
Original post by BrokenS0ulz
I think so too, I'm absolutely dreading it. I think my tactic is going to be spend a couple minutes on it and if I'm not getting anywhere then to skip it and come back at the end.
Good tactic
Bbc news has an article about a really difficult nmr in the ocr a paper (and paper in general) this year so I assume theyve got one for us too
Original post by suyoof123
Dont many ppl take f335, this thread is dead.
We need to bloat it so more people are here to impart their knowledge

Like here, what happens first.
A dipole is induced on chlorine by benzene, so the negative chloride reacts with alcl4?
and the positive chloride then accepts a pair of electrons from benzene to form a covalent bond?
We need to bloat it so more people are here to impart their knowledge


Like here, what happens first.
A dipole is induced on chlorine by benzene, so the negative chloride reacts with alcl4?
and the positive chloride then accepts a pair of electrons from benzene to form a covalent bond?
Not many schools in England take OCR B. Whats the mechanism for this reaction. Will we have to know the whole mechanism? If so, RIP to my grade.
Original post by EggsterminateMe
Not many schools in England take OCR B. Whats the mechanism for this reaction. Will we have to know the whole mechanism? If so, RIP to my grade.
I think we have to have an idea of the general mechanism, it applies to all of them so it's not too bad. I think the general mechanism is in the official revision guide
Does anyone have any tips for working out the structure of a molecule from NMR? Like any tips that just make it a whole lot easier? or am I just clutching at straws...
Original post by EggsterminateMe
Not many schools in England take OCR B. Whats the mechanism for this reaction. Will we have to know the whole mechanism? If so, RIP to my grade.
Electrophillic substitution iirc
chlorination bro ^^
Original post by suyoof123
Electrophillic substitution iirc
chlorination bro ^^
chlorination bro ^^
I know, but you see, I'm just memorising the reactions. I should've guess since benzene reaction undergo substitutions. Hydrogen is subsitituted for the chlorine?
Original post by Solarburst
I think we have to have an idea of the general mechanism, it applies to all of them so it's not too bad. I think the general mechanism is in the official revision guide
Does anyone have any tips for working out the structure of a molecule from NMR? Like any tips that just make it a whole lot easier? or am I just clutching at straws...
Does anyone have any tips for working out the structure of a molecule from NMR? Like any tips that just make it a whole lot easier? or am I just clutching at straws...
Ah thank you.
I read the CGP explanation that was helpful. Watch a video on it on youtube.
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- A Level Exam Discussions 2023
- GCSE Exam Discussions 2023
- OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]
- OCR Chemistry B (Salters) - Anybody doing this?
- GCSE Exam Discussions 2024
- AS and A level urgent help & advice
- chemistry
- A Level Chemistry (OCR B Salters Chemistry)
- AS/A Level Chemistry Study Group 2023/2024
- Revision website for OCR B (salters) chemistry
- How to get A/A* in A level chemistry
- A Level OCR Salters Chemistry B
- OCR B A-level Physics Paper 2 Advancing Physics (H557/02) - 9th June 2023 [Exam Chat]
- Is it possible to change sixth forms now (in year 12)?
- Politics or religious studies for A-level? Urgent!!
- A Level Chemistry Mark Scheme
- Good Computer Science University with average GCSEs and no FM?
- OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]
- I chose Computer Science GCSE OCR - help
Latest
Last reply 1 minute ago
University of Oxford 2025 Undergraduate Applicants Official ThreadLast reply 11 minutes ago
Even Europe’s far-right firebrands seem to sense Brexit is a disaster- The GuardianLast reply 16 minutes ago
Official Cambridge Postgraduate Applicants 2024 ThreadLast reply 16 minutes ago
ATAS (Academic, Technology, Approval Scheme) Certificate 2023/2024Last reply 19 minutes ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 hour ago
Government Social Research - Research Officer Scheme 2024Last reply 1 hour ago
LSE International Social and Public Policy and Economics (LLK1) 2024 ThreadPosted 1 hour ago
Failed 2 out of 3 exams semester 1 and scared about the 3 I have left in semester 2.Last reply 1 hour ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2927
Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]Trending
Last reply 1 week ago
AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]Last reply 1 week ago
AQA A-Level Chemistry Paper 1 (7405/1) - 10th June 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Chemistry Paper 1 Higher Tier Triple (8462 1H) - 17th May 2024 [Exam Chat]Last reply 1 week ago
OCR A-LEVEL CHEMISTRY PAPER 1 (H432/01) - 10th June [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 1 (Foundation Combined) 8464/1F - 17th May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 (Foundation Combined) 8464/2F - 11th June 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Chemistry Paper 2 Higher Tier Triple (8462 2H) - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 5 Chem 2 Foundation - 11th June 2024 [Exam Chat]Posted 3 weeks ago
Edexcel GCSE Combined Science Paper 2 Chem 1 Foundation - 17th May 2024 [Exam Chat]Last reply 2 months ago
Edexcel GCSE Combined Sci Paper 2 Higher Tier (1SC0 2CH) - 13th Jun 2023 [Exam Chat]



