AQA Physics PHYA5 - Thursday 18th June 2015 [Exam Discussion Thread]
Scroll to see replies
Original post by CD223
Q4 (c). June 2011. Why is the temperature higher and not lower? MS doesn't make sense to me that much :/
Q4 (c). June 2011. Why is the temperature higher and not lower? MS doesn't make sense to me that much :/
From what I remember,
the room temperature was higher than the initial temperature of the substance. Therefore the substance will take less time to change state and gain temperature until it is at room temperature (which is most of the time taken previously). After so, it will take longer to get to the final (previous / about 40) temperature in comparison to how long it previously took to get from room temperature to final temperature.
But since the time taken for final change in temperature (room to like 40) was insignificant in comparison to the time taken to get to room temperature (like -14 to room including state), it will take less time to get to 40 than the previous time. Therefore it will have extra time to heat up from the previous final temperature (like 40 or something).
(edited 8 years ago)
Original post by getback339
hi, could someone help with core 2014 question 1b ii. I know its a simple question im just getting myself confused, just not sure how to deal with the neutrons either side, thanks!
http://filestore.aqa.org.uk/subjects/AQA-PHYA51-QP-JUN14.PDF
http://filestore.aqa.org.uk/subjects/AQA-PHYA51-QP-JUN14.PDF
Anyone?
Original post by getback339
Anyone?
There are two ways to figure out the energy given off.
One way is to calculate the change in energy (mass defect) of both sides and see how much energy that converts to.
You cannot do this method in this case because you must account for the K.E (of the neutrons) as well as the masses of each side.
The other way to do it is find the change in binding energy of both sides.
Neutrons don't have a binding energy.
Original post by CD223
Q4 (c). June 2011. Why is the temperature higher and not lower? MS doesn't make sense to me that much :/
Q4 (c). June 2011. Why is the temperature higher and not lower? MS doesn't make sense to me that much :/
Haha how strange I just got stuck on the same question!! Do you get it yet because I can't see it?
Original post by betbi3etwerrd
There are two ways to figure out the energy given off.
One way is to calculate the change in energy (mass defect) of both sides and see how much energy that converts to.
You cannot do this method in this case because you must account for the K.E (of the neutrons) as well as the masses of each side.
The other way to do it is find the change in binding energy of both sides.
Neutrons don't have a binding energy.
One way is to calculate the change in energy (mass defect) of both sides and see how much energy that converts to.
You cannot do this method in this case because you must account for the K.E (of the neutrons) as well as the masses of each side.
The other way to do it is find the change in binding energy of both sides.
Neutrons don't have a binding energy.
But in june 14 you do it the first way ignoring the neutrons!?
Original post by AR_95
So charge has nothing to do with the fact that alpha is more ionising then Beta?
Isn't Alpha positive 2 charge, so more likely to attract the electrons out of the atom it collides with if we put aside momentum for now
Isn't Alpha positive 2 charge, so more likely to attract the electrons out of the atom it collides with if we put aside momentum for now
You're thinking is right only when alpha particles are not emitted towards any atoms or molecules. So in that case when alpha particles attract electrons, the electrons also attract alpha particles as stated by Newton. So in this case what is important is that which one of the particles will resist motion so that the other particle is moved towards the other particle. Electrons are attracted towards the nucleus of the atom. If the targeted atom has less protons than alpha particles then you would be right as the electrostatic force of attraction of alpha particles would overcome the electrostatic force of attraction of the nucleus of the atom towards the electron( hydrogen for example).
But usually this is not the case. When we talk about alpha particles we usually mean that they are traveling at a particular speed. Even though alpha particles have 2e charge their total mass is almost 7000 times greater than the mass of an electron. You could, for example, have 20 electrons as a particle (note that you can't join 20 electrons as a particle but this is an example for our purposes), fired at an atom to measure their ionising power. According to your reasoning this 20e particle would definitely beat alpha particles but if you think about it their mass is still 7000 less than the alpha particles and their momentum would easily be changed as they have less mass.
Posted from TSR Mobile
Edit: typing error
(edited 8 years ago)
Original post by Sbarron
But in june 14 you do it the first way ignoring the neutrons!?
No they do the second way, binding energy = [binding energy per nucleon x number of nucleons] for each side where neutrons have no binding energy?
Original post by betbi3etwerrd
No they do the second way, binding energy = [binding energy per nucleon x number of nucleons] for each side where neutrons have no binding energy?
Well I've just done it using mass finding the change in energy and ignoring neutrons and I got the correct answer
Original post by Sbarron
Well I've just done it using mass finding the change in energy and ignoring neutrons and I got the correct answer
I converted it to energy at the end
Original post by RobHunter97
Does anyone mind showing me how this would look please?
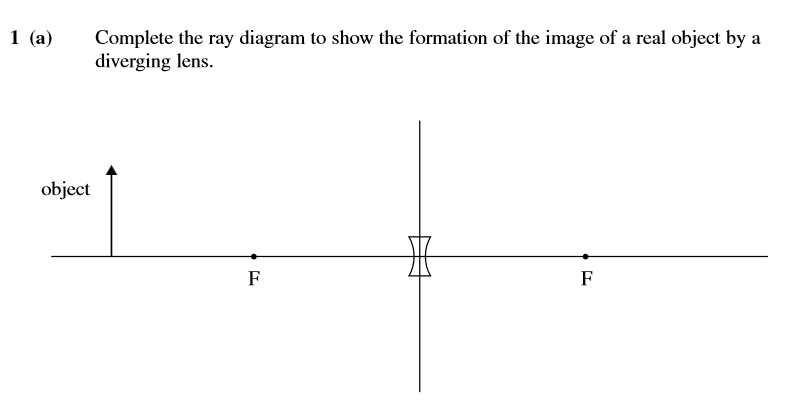
Mark scheme doesn't have an image to check against

Mark scheme doesn't have an image to check against
Any ideas?
Original post by Sbarron
Well I've just done it using mass finding the change in energy and ignoring neutrons and I got the correct answer
wait im looking at something else.. wait wait wait
Sorry ignore me... I did include the neutrons!! Getting tired now lol
Original post by Mehrdad jafari
You're thinking is right only when alpha particles are not emitted towards any atoms or molecules. So in that case when alpha particles attract electrons, the electrons also attract alpha particles as stated by Newton. So in this case what is important is that which one of the particles will resist motion so that the other particle is moved towards the other particle. Electrons are attracted towards the nucleus of the atom. If the targeted atom has less protons than alpha particles then you would be right as the electrostatic force of attraction of alpha particles would overcome the electrostatic force of attraction of the nucleus of the atom towards the electron( hydrogen for example).
But usually this is not the case. When we talk about alpha particles we usually mean that they are traveling at a particular speed. Even though alpha particles have 2e charge their total mass is almost 7000 times greater than the mass of an electron. You could, for example, have 20 electrons as a particle (note that you can't join 20 electrons as a particle but this is an example for our purposes), fired at an atom to measure their ionising power. According to your reasoning this 20e particle would definitely beat alpha particles but if you think about it their mass is still 7000 less than the alpha particles and their momentum would easily be changed as they have less mass.
Posted from TSR Mobile
Edit: typing error
But usually this is not the case. When we talk about alpha particles we usually mean that they are traveling at a particular speed. Even though alpha particles have 2e charge their total mass is almost 7000 times greater than the mass of an electron. You could, for example, have 20 electrons as a particle (note that you can't join 20 electrons as a particle but this is an example for our purposes), fired at an atom to measure their ionising power. According to your reasoning this 20e particle would definitely beat alpha particles but if you think about it their mass is still 7000 less than the alpha particles and their momentum would easily be changed as they have less mass.
Posted from TSR Mobile
Edit: typing error
Ok thanks for the explanation!
Original post by AR_95
Ok thanks for the explanation!
It's cool man
 . Glad you found it satisfying
. Glad you found it satisfying Posted from TSR Mobile
How does that even work
Posted from TSR Mobile
Original post by gcsestuff
The only think i can understand about those microscopes is that i cannot understand them lol
Posted from TSR Mobile
Ok so for the June 2014 energy released question this is how I done it using change in binding energy (B.e):

A question I have though is why in this (different) question do they do it using mass defect when they shouldn't have since the neutrons on the right hand side have large amounts kinetic energy which they do not account for!?
source : June 2011
I think the June 2011 question is wrong!
A question I have though is why in this (different) question do they do it using mass defect when they shouldn't have since the neutrons on the right hand side have large amounts kinetic energy which they do not account for!?
Attachment not found
source : June 2011
I think the June 2011 question is wrong!
(edited 8 years ago)
Original post by gcsestuff
Original post by Mehrdad jafari
The only think i can understand about those microscopes is that i cannot understand them lol
Posted from TSR Mobile
Posted from TSR Mobile
No way that's Turning points...

p.s the teacher guide is 1000x better then any stupid textbook, you were right
Original post by AR_95
No way that's Turning points... 
p.s the teacher guide is 1000x better then any stupid textbook, you were right

p.s the teacher guide is 1000x better then any stupid textbook, you were right
I haven't read the teacher's notes yet but i knew that's better. Those decided what to be written in our textbook think that we are mentally subnormal. In fact if you show the teacher your textbook he won't understand what's written in there.
Posted from TSR Mobile
Anyone have any good resources on optics and diverging and converging lenses for sight defects, what I'm reading might as well be written in arabic...
Quick Reply
Related discussions
- A-level Exam Discussions 2024
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- AQA A-Level Chemistry Paper 2 (7405/2) - 18th June 2024 [Exam Chat]
- GCSE Exam Discussions 2023
- OCR B A-level Physics Paper 2 Advancing Physics (H557/02) - 9th June 2023 [Exam Chat]
- AQA GCSE Physics Paper 2 (Foundation Combined) 8464/2F - 16th June 2023 [Exam Chat]
- AQA GCSE Physical sciences (Higher Combined) 8465/4H - 13th June 2023 [Exam Chat]
- OCR A-LEVEL CHEMISTRY A PAPER 2 (H432/02) - 21st June [Exam Chat]
- OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]
- OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]
- GCSE exam advice
- AQA A Level Physical Education Paper 1 7582/1 - 26 May 2022 [Exam Chat]
- AQA GCSE Food Preparation and Nutrition Paper 1 (8585/W) - 19th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 2 (8035/2) - 5th June 2024 [Exam Chat]
- AQA GCSE Geography Paper 3 (8035/3) - 14th June 2024 [Exam Chat]
- GCSE 2024 timetable
- AQA GCSE Chemistry Paper 2 Foundation Triple (8462 2F) - 11th June 2024 [Exam Chat]
- AQA GCSE Food Preparation and Nutrition - Tuesday 19th June 2024 [Exam Chat]
- English Lit Paper 2 leak??!?!!
Latest
Trending
Last reply 1 day ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]Trending
Last reply 1 day ago
AQA GCSE Physics Paper 1 (Higher Tier triple) 8463/1H - 22nd May 2024 [Exam Chat]Last reply 1 week ago
AQA GCSE Physics Paper 1 (Higher Combined) 8464/1H - 22nd May 2024 [Exam Chat]Posted 2 weeks ago
AQA GCSE Physics Paper 2 (Higher Tier triple) 8463/2H - 14th June 2024 [Exam Chat]Last reply 3 weeks ago
OCR A A-level Physics Paper 1 Modelling Physics (H556/01) - 24th May 2024 [Exam Chat]Last reply 1 month ago
AQA A-level Physics Paper 2 (7408/2) - 9th June 2023 [Exam Chat]Last reply 2 months ago
Edexcel A Level Physics Paper 3: 9PH0 03 - 15th June 2023 [Exam Chat]Last reply 3 months ago
AQA A-level Physics Paper 1 (7408/1) - 24th May 2023 [Exam Chat]Physics Exams
1190
Last reply 4 months ago
Edexcel GCSE Physics Paper 1 Higher Combined 1SC0 1PH - 25th May 2023 [Exam Chat]Last reply 5 months ago
Edexcel GCSE Physics Paper 1 Higher Tier Triple 1PH0 1H - 25th May 2023 [Exam Chat]Last reply 5 months ago
AQA A-level Physics Paper 3 (7408/3) - 15th June 2023 [Exam Chat]Last reply 10 months ago
Edexcel GCSE Physics Paper 2 Higher Tier Triple 1PH0 2H - 16th June 2023 [Exam Chat]Last reply 10 months ago
OCR B A-level Physics Paper 3 Advancing Physics (H557/03) - 15th Jun 2023 [Exam Chat]Last reply 10 months ago
OCR GCSE Physics A Paper 4 Higher Tier (J249/04) - 16th June 2023 [Exam Chat]