January Edexcel Chemistry IAL Unit 1 2016 (Thread)
Scroll to see replies
Original post by Zeekay98
What was the number of ions of Calcium in one mole of Calcium Hydroxide?
And the number of electrons in one mole of OH- ion?
And the number of electrons in one mole of OH- ion?
3 of moles of ions in 1 mol of Ca(OH)2
and 10 mols of electrons in 1 mol of OH- ion.
Original post by SAM RR
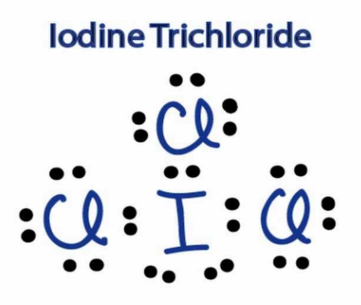
Can someone tell me apart from dispersion force are there any more inter molecular forces on iodine mono chloride? And how did you guys drew the LCL3 ?
ICl, has additional permanent dipole dipole forces, also it's electron number is greater. etc etc
ICl3 was like this, as Iodine can expand its d orbital accomodating more then 8 in valence.
(edited 8 years ago)
Original post by SaadKaleem
3 of moles of ions in 1 mol of Ca(OH)2
and 10 mols of electrons in 1 mol of OH- ion.
and 10 mols of electrons in 1 mol of OH- ion.
And what was the number of moles of iodine and bromine in the last question?
Original post by Zeekay98
And what was the number of moles of iodine and bromine in the last question?
I got that question wrong

but the answer is 0.17 mol of Br2 and 0.33 mol of I2
Original post by SaadKaleem
I got that question wrong 
but the answer is 0.17 mol of Br2 and 0.33 mol of I2

but the answer is 0.17 mol of Br2 and 0.33 mol of I2
Ahh crap. I'm definitely losing a lot of marks in section C.
Since I'm a private candidate, I didn't get to talk to anyone about their paper. But do you know how it was for other people?
Original post by Zeekay98
Ahh crap. I'm definitely losing a lot of marks in section C.
Since I'm a private candidate, I didn't get to talk to anyone about their paper. But do you know how it was for other people?
Since I'm a private candidate, I didn't get to talk to anyone about their paper. But do you know how it was for other people?
About 15 gave from my centre, they found the paper to be of slight hard difficulty.
In my opinion, it was an average paper.
(edited 8 years ago)
Original post by Raghdaelgedawy
Economy was 82 percent and yield was seventy something ( 73.5 i think
Bulls-eye. Got that on the point matE!
Original post by SaadKaleem
About 15 gave from my centre, they found the paper to be of slight hard difficulty.
In my opinion, it was an average paper.
In my opinion, it was an average paper.
True. I just hope the curve is good. Anyways, thanks for not ignoring my messages!
 haha
hahaOriginal post by SaadKaleem
3 of moles of ions in 1 mol of Ca(OH)2
and 10 mols of electrons in 1 mol of OH- ion.
and 10 mols of electrons in 1 mol of OH- ion.
Weren't we supposed to multiply avagadros constant with it?
Original post by Zeekay98
True. I just hope the curve is good. Anyways, thanks for not ignoring my messages!  haha
haha
 haha
hahaHey, what was your answer for the h2so4 to so2 equation????
Original post by elhm18
Weren't we supposed to multiply avagadros constant with it?
No, it said 'mol' of electrons, not 'number' of electrons.
Original post by elhm18
Hey, what was your answer for the h2so4 to so2 equation????
You mean Sulfuric Acid (and KI) to Sulfur Ionic equation?
Original post by Zeekay98
Ahh crap. I'm definitely losing a lot of marks in section C.
Since I'm a private candidate, I didn't get to talk to anyone about their paper. But do you know how it was for other people?
Since I'm a private candidate, I didn't get to talk to anyone about their paper. But do you know how it was for other people?
Dont worry my exam was pretty crap. Section C and then a few of Section B. You want to discuss them?
Original post by dnac5
Dont worry my exam was pretty crap. Section C and then a few of Section B. You want to discuss them?
What was the question regarding the Ionic equation? on the 2nd page of section C, i forgot.
Original post by SaadKaleem
What was the question regarding the Ionic equation? on the 2nd page of section C, i forgot.
It was the equation for potassium iodide and sulfuric acid. and then you had to give an ionic equation on how sulfur is produced from H2SO4. BUT then i went and looked in my book after the exam, and suprisingly it said that the equation of KI and H2SO4 is not required for A-Level, so my teacher is submitting a complaint.
Did you get the dot and cross diagram for ICL3 right?
and what about that question of the preparation of the CACO3 from CaOH?
Original post by dnac5
Dont worry my exam was pretty crap. Section C and then a few of Section B. You want to discuss them?
Well for me section B was pretty good except I might've messed up the CaCO3 decomposition question a bit.
Section C started off fine but then the rest of the questions were weird.
I really think we should leave this here & focus on the remaining ones!
 haha
hahaFingers crossed! Hope the curve is good
Original post by Zeekay98
Well for me section B was pretty good except I might've messed up the CaCO3 decomposition question a bit.
Section C started off fine but then the rest of the questions were weird.
I really think we should leave this here & focus on the remaining ones! haha
haha
Fingers crossed! Hope the curve is good
Section C started off fine but then the rest of the questions were weird.
I really think we should leave this here & focus on the remaining ones!
 haha
hahaFingers crossed! Hope the curve is good
Ive got only my unit 3 left, fingers crossed for that one
Original post by dnac5
Ive got only my unit 3 left, fingers crossed for that one
Oh same!
Since this unit is all based on Unit 1 & 2 practicals, I couldn't find any sort of revision notes for this.
How are you revising?
Original post by dnac5
It was the equation for potassium iodide and sulfuric acid. and then you had to give an ionic equation on how sulfur is produced from H2SO4. BUT then i went and looked in my book after the exam, and suprisingly it said that the equation of KI and H2SO4 is not required for A-Level, so my teacher is submitting a complaint.
Did you get the dot and cross diagram for ICL3 right?
and what about that question of the preparation of the CACO3 from CaOH?
Did you get the dot and cross diagram for ICL3 right?
and what about that question of the preparation of the CACO3 from CaOH?
Yes, I got the ICl3 right, i posted above.
For the ionic equation, i wrote this:
H2SO4 + 6H+ + 6e- --> 4H2O + S
---
Prep of slaked lime (Ca(OH2) from CaCO3 (Two stages)
I wrote, CaCO3 --> CaO + CO2 and CaO + H2O --> Ca(OH)2 and high temp condition etc.
(edited 8 years ago)
Original post by Zeekay98
Oh same!
Since this unit is all based on Unit 1 & 2 practicals, I couldn't find any sort of revision notes for this.
How are you revising?
Since this unit is all based on Unit 1 & 2 practicals, I couldn't find any sort of revision notes for this.
How are you revising?
I studied for it last year, im just re sitting it as i got a B, and im trying to get a higher grade. I studied from the Unit 3 & & edexcel book. But I did notes on them, if you want them, they're hand written though :/ Any questions i can help you wit, as i have a bit of experience on it
Quick Reply
Related discussions
- Official thread - January 2023 IAL edexcel
- Edexcel IAL Business Studies Notes
- IAL repeats cash in.
- A-level Exam Discussions 2024
- chemistry alevel mock
- What units do I need for Edexcel IAL or IAS Further Maths
- Edexcel ial information technology
- Edexcel Past Papers
- A-level Business Study Group 2022-2023
- A Level Advice
- IAL Edexcel recent past papers
- GCSE Exam Discussions 2024
- A Level Exam Discussions 2023
- Edexcel IAL units
- Physics - i need a miracle
- Ial chemistry
- Past papers for IAL Information Technology Unit 1 (WIT11/01) and Unit 2 (WIT12/01)
- Edexcel IAL BIOLOGY JANUARY 2024
- Edexcel IAL results
- Edexcel AS level unit-1 and unit-2 business notes
Latest
Last reply 1 minute ago
Official London School of Economics and Political Science 2024 Applicant ThreadLast reply 1 minute ago
Anyone else get an offer for a Rolls Royce degree apprenticeship in Derby (2024)?Last reply 3 minutes ago
Amazon apprenticeship 2024 offer holdersLast reply 4 minutes ago
Official Dental Hygiene and Therapy (Oral Health Science) 2024 Entry ThreadDentistry
2910
Last reply 15 minutes ago
Why is the political left now censorious and authoritarian??Last reply 16 minutes ago
OCR A-level Religious Studies Paper 3 (Christianity) - 20th June 2024 [Exam Chat]Last reply 18 minutes ago
IBM Degree Apprenticeship 2024Trending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 4 days ago
Im confused about this chemistry question, why does it form these products



