A-level Chemistry Revision Squad!
Scroll to see replies
Original post by olivia7001
Why doesn't Kc change if you change any concentrations or pressure at equilibrium? It only changes with temperature change.
surely concentrations of products and reactants change so Kc would change?
surely concentrations of products and reactants change so Kc would change?
Follow me on this:
Kc is a constant, right? And the general equation is:
Let's say that the conc. of c increases. Because the left hand side of the equation must be constant (kc has to be kept the same), the top of the fraction must change in order to account for the change in conc of c. Therefore, even though the conc. of c has changed, the conc. of the other reactants will change, rather than kc changing.
Pressure is just the conc. in a given volume. so it works by the same principle stated above.
Have a look at the mindmap for more kinetics stuff. Hope that helps.
(edited 8 years ago)
Original post by PlayerBB
If we change the concentration or pressure, Kc/Kp initially change because according to Chatelier principle, the P.O.E will shift to the side that oppose the change to restore equilibrium, so when we shift to a side, the new Kc/Kp value is smaller or bigger depending to which side was shifted then when equilibrium is restored, the value of Kc/Kp is restored
Posted from TSR Mobile
Posted from TSR Mobile
What is Kp ?

Original post by FemaleBo55
Can someone please help me with this question:
A mixture of hydrogen of concentration 10moldm^-3 and CO2 of concentration 90moldm^-3 was heated at 1200K so that steam and CO was produced. At equilibrium only 80.53moldm^-3 od CO2 remained in the mixture. Calculate Kc for the reaction.
So far I have the expression for it: Kc = [CO][H2O] / [CO2]
I'm not sure if I did this right but I did: 90 - 80.53 to give me 9.47 and I think this means that only 9.47 of CO2 was reacted with H2. Not sure if thats correct?
I have no clue what to do after this? I know that I have to find the concetration for bot hof the products formed but how.. ://
Help appreciated
A mixture of hydrogen of concentration 10moldm^-3 and CO2 of concentration 90moldm^-3 was heated at 1200K so that steam and CO was produced. At equilibrium only 80.53moldm^-3 od CO2 remained in the mixture. Calculate Kc for the reaction.
So far I have the expression for it: Kc = [CO][H2O] / [CO2]
I'm not sure if I did this right but I did: 90 - 80.53 to give me 9.47 and I think this means that only 9.47 of CO2 was reacted with H2. Not sure if thats correct?
I have no clue what to do after this? I know that I have to find the concetration for bot hof the products formed but how.. ://
Help appreciated

Hi- I've given it a shot apologies if there are any mistakes...
Here goes:
So yes, I did the same thing I did 90-80.53= 9.47 then (using the RICE) method I did the same with (H2). So I did 10-9.47= 0.43moldm-3.
Then on the other side of the equation we are not told the concentration of CO or H20 so I assumed it was 0 for both. So the conc of both CO and H20 would be 9.47. (0+9.47=9.47)
Then using the Kc equation I multiplied (9.47 x 9.47)/ (0.53 x 80.53)= 2.1
Kc = 2.1 (i think)
Original post by P____P
Follow me on this:
Kc is a constant, right? And the general equation is:
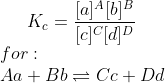
Let's say that the conc. of c increases. Because the left hand side of the equation must be constant (kc has to be kept the same), the top of the fraction must change in order to account for the change in conc of c. Therefore, even though the conc. of c has changed, the conc. of the other reactants will change, rather than kc changing.
Pressure is just the conc. in a given volume. so it works by the same principle stated above.
Have a look at the mindmap for more kinetics stuff. Hope that helps.
Kc is a constant, right? And the general equation is:
Let's say that the conc. of c increases. Because the left hand side of the equation must be constant (kc has to be kept the same), the top of the fraction must change in order to account for the change in conc of c. Therefore, even though the conc. of c has changed, the conc. of the other reactants will change, rather than kc changing.
Pressure is just the conc. in a given volume. so it works by the same principle stated above.
Have a look at the mindmap for more kinetics stuff. Hope that helps.
So is it that proportionally everything changes by the same amount, so the factor that everything changed by ends up cancelling out in the fraction?
Original post by haj101
Hi- I've given it a shot apologies if there are any mistakes...
Here goes:
So yes, I did the same thing I did 90-80.53= 9.47 then (using the RICE) method I did the same with (H2). So I did 10-9.47= 0.43moldm-3.
Then on the other side of the equation we are not told the concentration of CO or H20 so I assumed it was 0 for both. So the conc of both CO and H20 would be 9.47. (0+9.47=9.47)
Then using the Kc equation I multiplied (9.47 x 9.47)/ (0.53 x 80.53)= 2.1
Kc = 2.1 (i think)
Here goes:
So yes, I did the same thing I did 90-80.53= 9.47 then (using the RICE) method I did the same with (H2). So I did 10-9.47= 0.43moldm-3.
Then on the other side of the equation we are not told the concentration of CO or H20 so I assumed it was 0 for both. So the conc of both CO and H20 would be 9.47. (0+9.47=9.47)
Then using the Kc equation I multiplied (9.47 x 9.47)/ (0.53 x 80.53)= 2.1
Kc = 2.1 (i think)
Thanks I get that now!
 But is the RICE method? I've never heard of it LOL -.-
But is the RICE method? I've never heard of it LOL -.-Original post by FemaleBo55
Thanks I get that now!  But is the RICE method? I've never heard of it LOL -.-
But is the RICE method? I've never heard of it LOL -.-
 But is the RICE method? I've never heard of it LOL -.-
But is the RICE method? I've never heard of it LOL -.-I'm glad you understand!

It's basically:
Ratio
Initial moles
Change
Equilibrium moles
(It allows me to find the equilibrium moles a lot quicker and easier!)
Original post by P____P
What is Kp ?


Pressure equilibrium constant
Original post by thatcooldude2.0
What uni are you at? How are you finding the course in general and what is the most interesting topic you've done so far? 

I'm at uea doing their mchem degree. Honestly, its great, the lecturers are all really nice, they clearly know their stuff and put the students first. The first year really is just to get everyone on the same level, which means the content doesnt come as heavy as you might think, they are also a bit more lenient about the quality of lab reports and things in the first year. Come second year they expect you to pull your socks up and start throwing a hell of a lot of knowledge your way, especially in physical chemistry, but that just adds to the excitement I feel. My favourite topic so far has been frustrated lewis pairs, the general concept of which is you take a strong lewis acid (eg.a borane) And a strong lewis base (an amine) and stearically hinder them so they cant react. You can then pass small molecules such as hydrogen gas through the mixture, and the force between the lewis pair rips the H2 molecule apart, allowing the extraction of the electrons into a battery of some form.
I hope this helped, sorry about grammar, im typing this on a tablet

Original post by Spudgunhimself
I'm at uea doing their mchem degree. Honestly, its great, the lecturers are all really nice, they clearly know their stuff and put the students first. The first year really is just to get everyone on the same level, which means the content doesnt come as heavy as you might think, they are also a bit more lenient about the quality of lab reports and things in the first year. Come second year they expect you to pull your socks up and start throwing a hell of a lot of knowledge your way, especially in physical chemistry, but that just adds to the excitement I feel. My favourite topic so far has been frustrated lewis pairs, the general concept of which is you take a strong lewis acid (eg.a borane) And a strong lewis base (an amine) and stearically hinder them so they cant react. You can then pass small molecules such as hydrogen gas through the mixture, and the force between the lewis pair rips the H2 molecule apart, allowing the extraction of the electrons into a battery of some form.
I hope this helped, sorry about grammar, im typing this on a tablet
I hope this helped, sorry about grammar, im typing this on a tablet

Thanks for the reply, that sounds really interesting!
 Do you like the uni itself? Also what are you thinking of doing when you finish your degree?
Do you like the uni itself? Also what are you thinking of doing when you finish your degree?Original post by olivia7001
So is it that proportionally everything changes by the same amount, so the factor that everything changed by ends up cancelling out in the fraction?
Yup, that's one (mathematical) way of looking at it. Also remember that chemically 'the equilibrium position shifts to opposite side, to oppose the change in pressure/volume or conc.'
That phrase works basically every time in AS and A2 on AQA spec.

(edited 8 years ago)
Original post by haj101
I'm glad you understand! 
It's basically:
Ratio
Initial moles
Change
Equilibrium moles
(It allows me to find the equilibrium moles a lot quicker and easier!)

It's basically:
Ratio
Initial moles
Change
Equilibrium moles
(It allows me to find the equilibrium moles a lot quicker and easier!)
Okay so for my question lets see if I understand it using your method lol:
Ratio for reactants is 1:1 and for the products is also 1:1
Initial moles: they haven't given me any moles so I'm just gunna use concentration, is that okay? so initial conc of: H2 = 10moldm-3, CO2 = 90moldm-3 and for the products is 0 because the reaction hasnt started yet?
Change: from the question we know that 80.53 of CO2 remained so from 90 only 9.47 of reacted with H2. As the ratio is one to one same the amount of H2 must have been reacted too hence 10 - 9.47 = 0.53?
WIth regards to the prodcuts as both are 1:1 ration with respect to product:reactants the product must have increased by 9.47 if the reactants decreased by 9.47? Therefore both H2O and CO are 9.47moldm-3
Equilibrium mole: not sure what you write here? LOL
Then for Kc you just plug them in the formula?

Original post by P____P
Follow me on this:
Kc is a constant, right? And the general equation is:
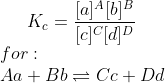
Let's say that the conc. of c increases. Because the left hand side of the equation must be constant (kc has to be kept the same), the top of the fraction must change in order to account for the change in conc of c. Therefore, even though the conc. of c has changed, the conc. of the other reactants will change, rather than kc changing.
Pressure is just the conc. in a given volume. so it works by the same principle stated above.
Have a look at the mindmap for more kinetics stuff. Hope that helps.
Kc is a constant, right? And the general equation is:
Let's say that the conc. of c increases. Because the left hand side of the equation must be constant (kc has to be kept the same), the top of the fraction must change in order to account for the change in conc of c. Therefore, even though the conc. of c has changed, the conc. of the other reactants will change, rather than kc changing.
Pressure is just the conc. in a given volume. so it works by the same principle stated above.
Have a look at the mindmap for more kinetics stuff. Hope that helps.
Lol I really like your mind maps, what other ones have you made for chemistry?

Original post by FemaleBo55
Okay so for my question lets see if I understand it using your method lol:
Ratio for reactants is 1:1 and for the products is also 1:1
Initial moles: they haven't given me any moles so I'm just gunna use concentration, is that okay? so initial conc of: H2 = 10moldm-3, CO2 = 90moldm-3 and for the products is 0 because the reaction hasnt started yet?
Change: from the question we know that 80.53 of CO2 remained so from 90 only 9.47 of reacted with H2. As the ratio is one to one same the amount of H2 must have been reacted too hence 10 - 9.47 = 0.53?
WIth regards to the prodcuts as both are 1:1 ration with respect to product:reactants the product must have increased by 9.47 if the reactants decreased by 9.47? Therefore both H2O and CO are 9.47moldm-3
Equilibrium mole: not sure what you write here? LOL
Then for Kc you just plug them in the formula?
Ratio for reactants is 1:1 and for the products is also 1:1
Initial moles: they haven't given me any moles so I'm just gunna use concentration, is that okay? so initial conc of: H2 = 10moldm-3, CO2 = 90moldm-3 and for the products is 0 because the reaction hasnt started yet?
Change: from the question we know that 80.53 of CO2 remained so from 90 only 9.47 of reacted with H2. As the ratio is one to one same the amount of H2 must have been reacted too hence 10 - 9.47 = 0.53?
WIth regards to the prodcuts as both are 1:1 ration with respect to product:reactants the product must have increased by 9.47 if the reactants decreased by 9.47? Therefore both H2O and CO are 9.47moldm-3
Equilibrium mole: not sure what you write here? LOL
Then for Kc you just plug them in the formula?

Yes!! We can apply it to conc questions... For the equilibrium mole we put the calculated conc (from the change) and the equlibrium conc/moles is used in the equation.
Aww Im happy you understand!

Anyone doing edexcel?
Posted from TSR Mobile
Posted from TSR Mobile
Original post by kiiten
Could someone please clarify if this is right please?
Is (C2H5)2C(OH)CH3 2-ethyl-butan-2-ol
Is (C2H5)2C(OH)CH3 2-ethyl-butan-2-ol
Yes you are correct.

Original post by jyyl
Yes you are correct. 

Thanks - I wasn't sure if it was ethyl or methyl

Original post by kiiten
Thanks - I wasn't sure if it was ethyl or methyl 

The longest carbon chain has 4 carbon atoms and the side chain is C2H5 so it's ethyl. If the side chain is CH3 instead of C2H5, then it would be methyl.
Original post by jyyl
The longest carbon chain has 4 carbon atoms and the side chain is C2H5 so it's ethyl. If the side chain is CH3 instead of C2H5, then it would be methyl.
Yeah, I wasn't sure if I was counting the longest chain or not. I have another question:
Is the structural formula for pentanoic acid
CH3CH2CH2C=OCH2(OH) - I'm not sure if there are meant to be brackets on the O or if you show the = bond
Original post by kiiten
Yeah, I wasn't sure if I was counting the longest chain or not. I have another question:
Is the structural formula for pentanoic acid
CH3CH2CH2C=OCH2(OH) - I'm not sure if there are meant to be brackets on the O or if you show the = bond
Is the structural formula for pentanoic acid
CH3CH2CH2C=OCH2(OH) - I'm not sure if there are meant to be brackets on the O or if you show the = bond
For pentanoic acid, write like this will do
 CH3CH2CH2CH2COOH
CH3CH2CH2CH2COOHQuick Reply
Related discussions
- TSR Study Together - STEM vs Humanities!
- GCSE Exam Discussions 2024
- A-level Chemistry Help
- A-level chemistry revision, taking notes and exam practise help and paper 3
- A- levels
- A level chemistry
- Where to revise for module 1 for OCR A chemistry?
- Im failing a-level chemistry! Help!
- Chemistry A-level
- Is SnapRevise for chemistry worth the cost ?
- when did u start revising for a levels?
- Chemistry A-level?
- Should i drop chemistry
- Learning techniques
- A- levels
- Revision for A level
- Revision tips for Year 12s? Biology, Chemistry, Maths.
- I've forgotten everything in A level chemistry
- Free revision/quizzez to improve my grades for A lvl Bio and Chem
- A level choice dilema!!!
Latest
Trending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 6 days ago
Im confused about this chemistry question, why does it form these products



