Proton NMR What am I missing?
I honestly feel I am missing something major and therefore can't finish this question off, which I think would be essential to all NMR questions.
Okay, I got most of the vital info but I just don't know which to choose?
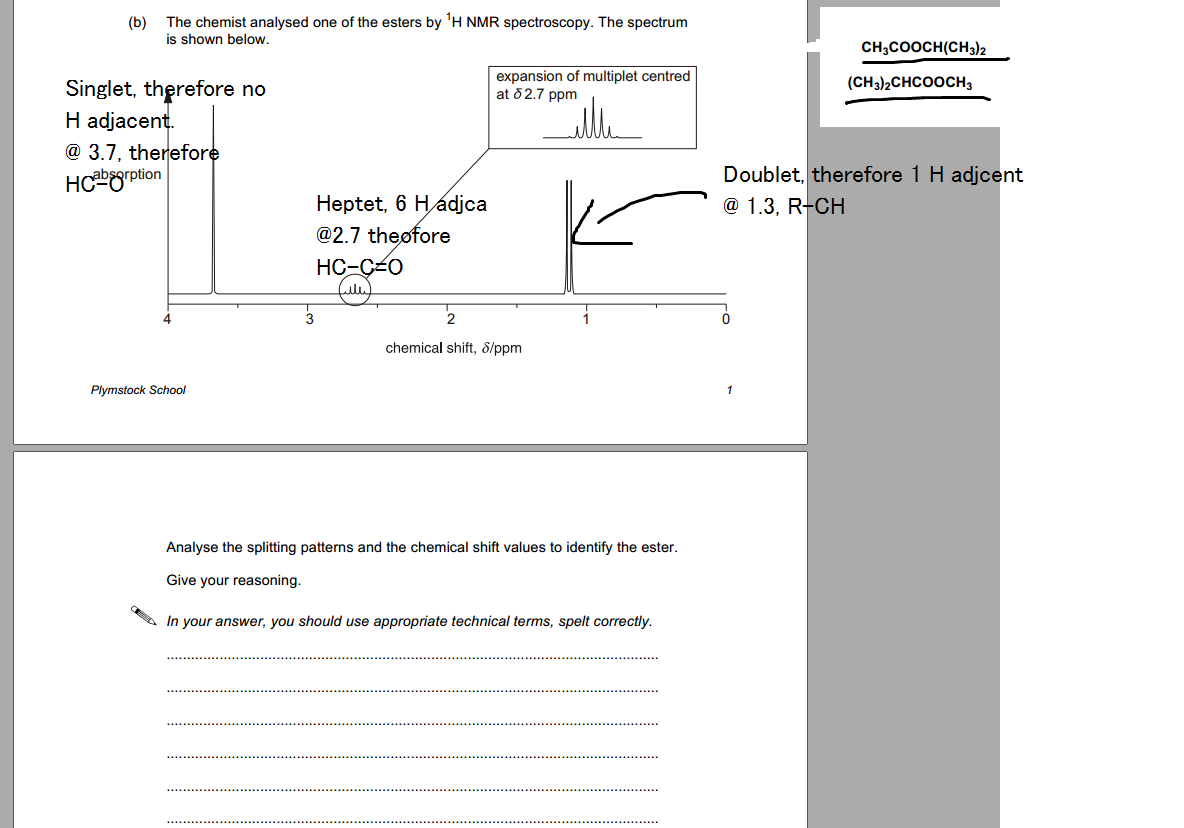
Okay, I got most of the vital info but I just don't know which to choose?

Scroll to see replies
Original post by Lawbringer
I honestly feel I am missing something major and therefore can't finish this question off, which I think would be essential to all NMR questions.
Okay, I got most of the vital info but I just don't know which to choose?

Okay, I got most of the vital info but I just don't know which to choose?

Can you show the whole question?
Original post by alow
Can you show the whole question?


Original post by Lawbringer
I honestly feel I am missing something major and therefore can't finish this question off, which I think would be essential to all NMR questions.
Okay, I got most of the vital info but I just don't know which to choose?

Okay, I got most of the vital info but I just don't know which to choose?

Hi, I don't think you're missing anything. The spectrum must be for ester (CH3)2CHCOOCH3. Distinguishing peak is the one split into 7 at 2.7ppm. (It's HC-C=O adjacent to 6 Hs). This (multiplet) wouldn't be present in the HNMR spectrum of the other ester (the peak at 2.7ppm would be a singlet because H in HC-C=O environment is not adjacent to any protons). Try and draw the structures out. Hope this helps

Original post by Lawbringer



The shift for the 3 identical methyl protons is pretty high, what can you deduce from that?
Original post by Terrificmagenta
x
There are two esters which match the splitting patterns. You also need to use the shifts.
Original post by alow
There are two esters which match the splitting patterns. You also need to use the shifts.
Which ones??

Original post by Terrificmagenta
Which ones?? 

(CH3)2CHCOOCH3 and CH3COOCH(CH3)2.
Original post by alow
The shift for the 3 identical methyl protons is pretty high, what can you deduce from that?
I have no idea what you mean? This topic is killing me. I just can't finish the questions or form the actual finished products. have you got any good resources? The OCR book is so bad at explaining this, all their questions in examples are the easiest ones ever lol /rant
I really can't make sense of how high a chemical shift can mean something?
Original post by alow
(CH3)2CHCOOCH3 and CH3COOCH(CH3)2.
No, the splitting for CH3COOCH(CH3)2 will be different. There would be no multiplet at 2.7ppm. It would be a singlet
Original post by Lawbringer
I have no idea what you mean? This topic is killing me. I just can't finish the questions or form the actual finished products. have you got any good resources? The OCR book is so bad at explaining this, all their questions in examples are the easiest ones ever lol /rant
I really can't make sense of how high a chemical shift can mean something?
I really can't make sense of how high a chemical shift can mean something?
Unless you want to know a bit more about NMR, I can't think of anything. If you do, there's some Oxford Chemistry Primers that are pretty good.
High chemical shift means that the proton's (or whatever NMR you're doing) is highly deshielded (i.e. there's less electron density around the proton). What could be a cause of this?
Original post by Terrificmagenta
No, the splitting for CH3COOCH(CH3)2 will be different. There would be no multiplet at 2.7ppm. It would be a singlet
It would be a septet. There is 3J coupling between the single proton and the six protons of the two methyl groups it is next to.
(edited 8 years ago)
Original post by Lawbringer
I have no idea what you mean? This topic is killing me. I just can't finish the questions or form the actual finished products. have you got any good resources? The OCR book is so bad at explaining this, all their questions in examples are the easiest ones ever lol /rant
I really can't make sense of how high a chemical shift can mean something?
I really can't make sense of how high a chemical shift can mean something?
I don't think you need to know anything about the significance of a high shift for the OCR spec
 . Dw about it, you can still pick up majority of the marks even if you don't get the final structure!
. Dw about it, you can still pick up majority of the marks even if you don't get the final structure!  I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments
I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments 
Original post by Terrificmagenta
I don't think you need to know anything about the significance of a high shift for the OCR spec  . Dw about it, you can still pick up majority of the marks even if you don't get the final structure!
. Dw about it, you can still pick up majority of the marks even if you don't get the final structure!  I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments
I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments 
 . Dw about it, you can still pick up majority of the marks even if you don't get the final structure!
. Dw about it, you can still pick up majority of the marks even if you don't get the final structure!  I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments
I feel like a parrot rn coz i always hear my teacher say that to my friends haha. As long as you can use the n+1 rule and figure out the environments 
Well that's just terrible advice.
Original post by alow
It would be a septet. There is 3J coupling between the single proton and the six protons of the two methyl groups it is next to.
There arent any adjacent protons in the environment thoughh lol
(edited 8 years ago)
Draw the displayed formulas for all the esters. Look at splitting patterns
Original post by Terrificmagenta
There arent any adjacent protons in the environment thoughh lol
Yes there are... Look again.
Original post by Serine Soul
Draw the displayed formulas for all the esters. Look at splitting patterns
Yeah if you see the 1st pic, I actually did that. It's just the final marks I really can't get, and I don't wish to settle with 7/8 lol. I really want to ace it haha
Original post by Lawbringer
Yeah if you see the 1st pic, I actually did that. It's just the final marks I really can't get, and I don't wish to settle with 7/8 lol. I really want to ace it haha
All you need to do is decide which of the singlet and multiplet would be more deshielded in either compound.
Original post by alow
Yes there are... Look again.
Quick Reply
Related discussions
- How do you do NMR questions!?
- A level chemistry
- NMR Calculator/predictor?
- Help chemistry urgent
- Edexcel A Level Chemistry Paper 3 2023
- Spectroscopy help
- Help spectroscopy
- Chemistry help
- NMR question
- AQA A level bio help?
- AQA a level physics question - beta minus decay
- The A-level grind
- what do the empty sticks mean in the chemistry data sheet?
- Specific Charge
- Mass vs relative atomic mass on periodic table
- Chemistry - NMR
- Alevel physics
- Chem - PH Question
- AQA A Level Chemistry
- SPecific charge - physics homework
Latest
Trending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these productsTrending
Last reply 1 week ago
Im confused about this chemistry question, why does it form these products



